Details of the Target
General Information of Target
| Target ID | LDTP05013 | |||||
|---|---|---|---|---|---|---|
| Target Name | Small ribosomal subunit protein eS25 (RPS25) | |||||
| Gene Name | RPS25 | |||||
| Gene ID | 6230 | |||||
| Synonyms |
Small ribosomal subunit protein eS25; 40S ribosomal protein S25 |
|||||
| 3D Structure | ||||||
| Sequence |
MPPKDDKKKKDAGKSAKKDKDPVNKSGGKAKKKKWSKGKVRDKLNNLVLFDKATYDKLCK
EVPNYKLITPAVVSERLKIRGSLARAALQELLSKGLIKLVSKHRAQVIYTRNTKGGDAPA AGEDA |
|||||
| Target Bioclass |
Other
|
|||||
| Family |
Eukaryotic ribosomal protein eS25 family
|
|||||
| Subcellular location |
Cytoplasm
|
|||||
| Function | Component of the small ribosomal subunit. The ribosome is a large ribonucleoprotein complex responsible for the synthesis of proteins in the cell. | |||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
m-APA Probe Info |
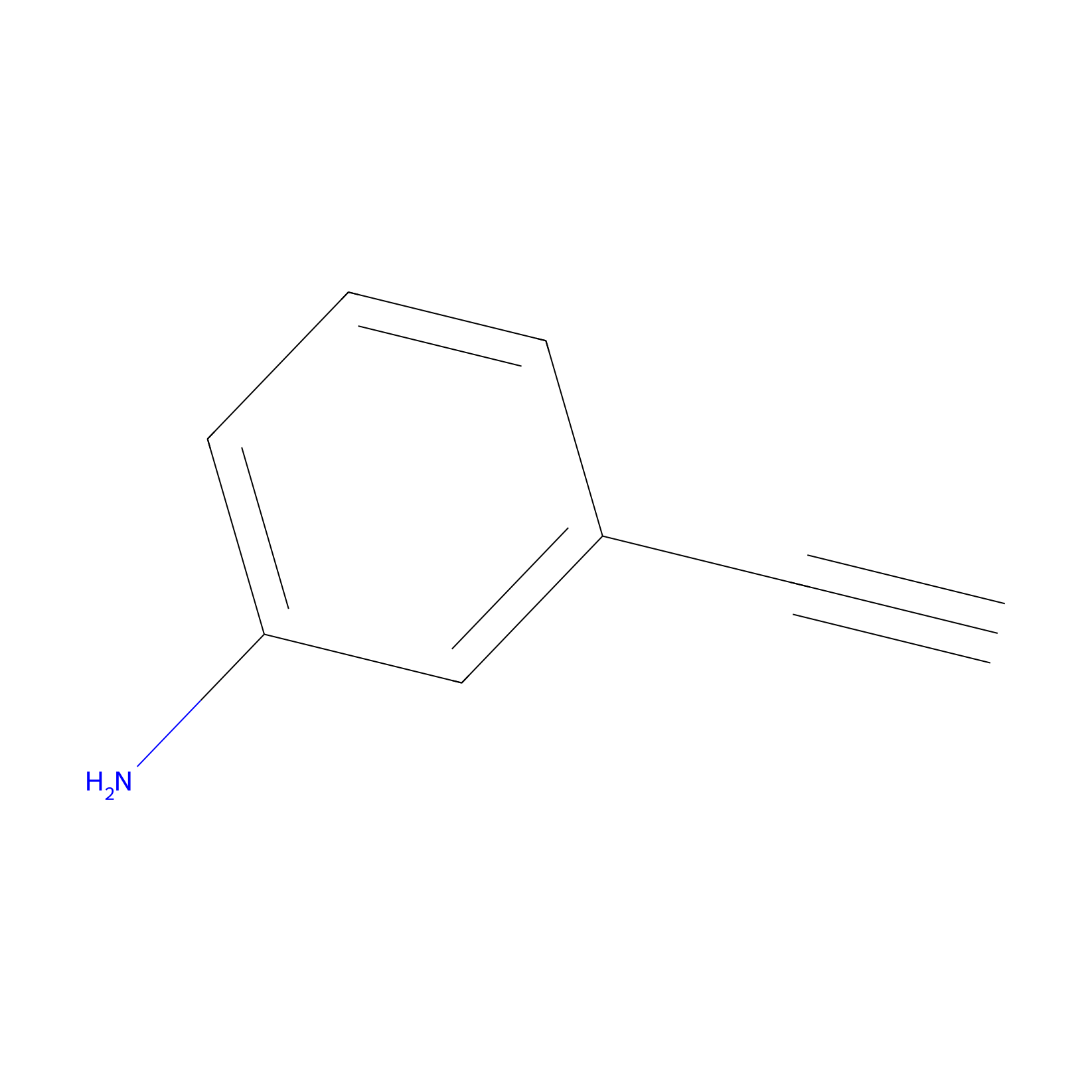 |
9.12 | LDD0402 | [1] | |
|
A-EBA Probe Info |
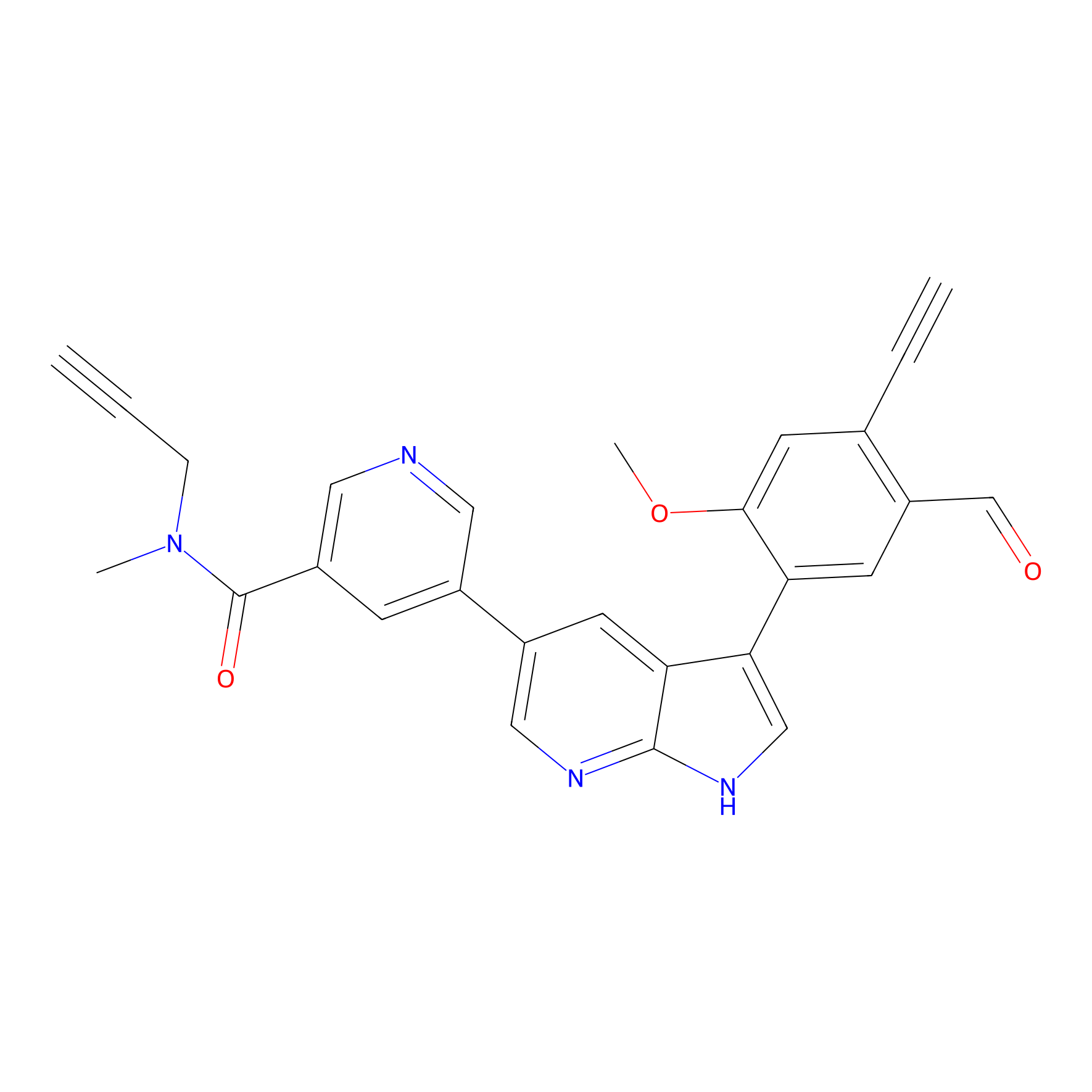 |
3.35 | LDD0215 | [2] | |
|
CY4 Probe Info |
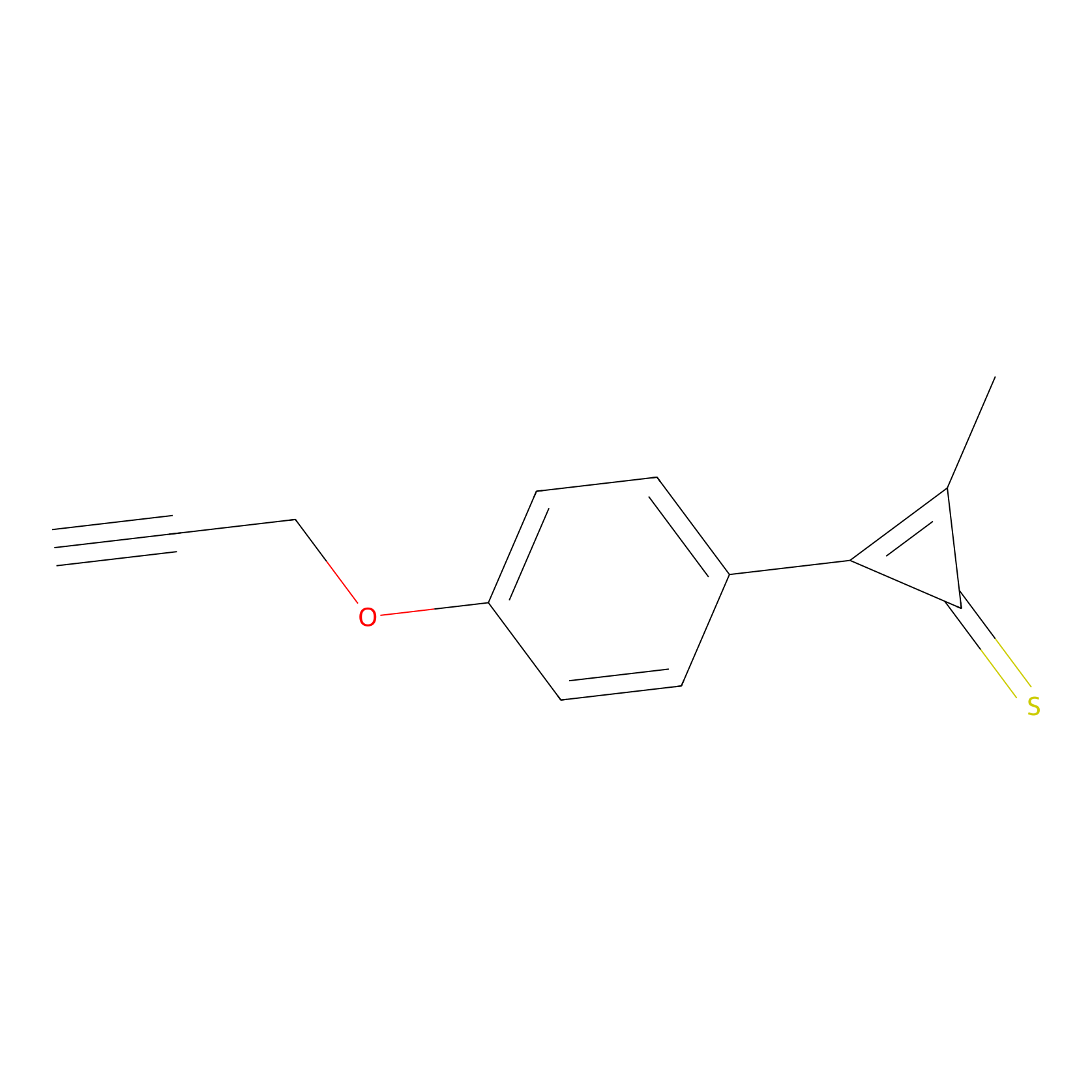 |
8.86 | LDD0244 | [3] | |
|
N1 Probe Info |
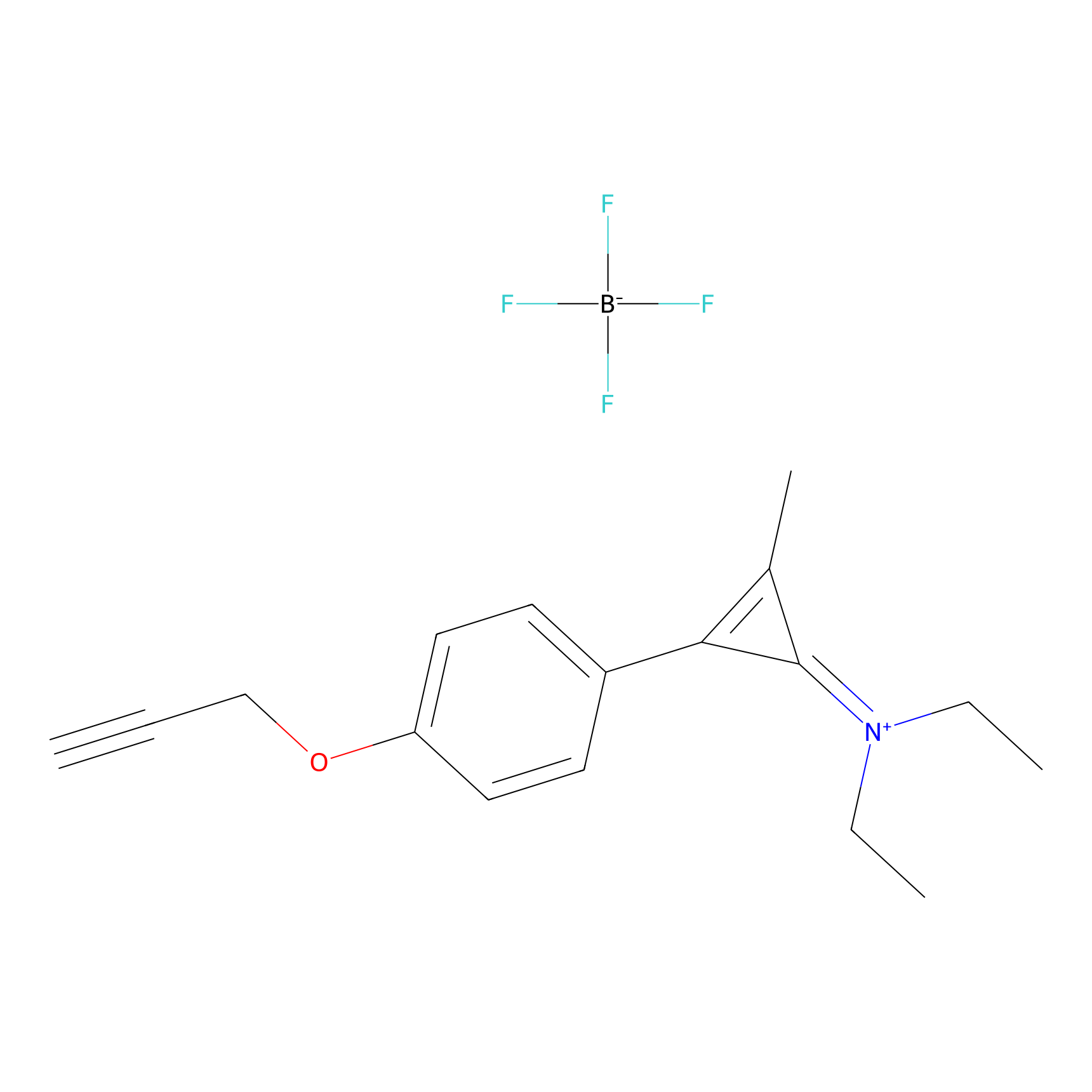 |
5.92 | LDD0242 | [3] | |
|
TH211 Probe Info |
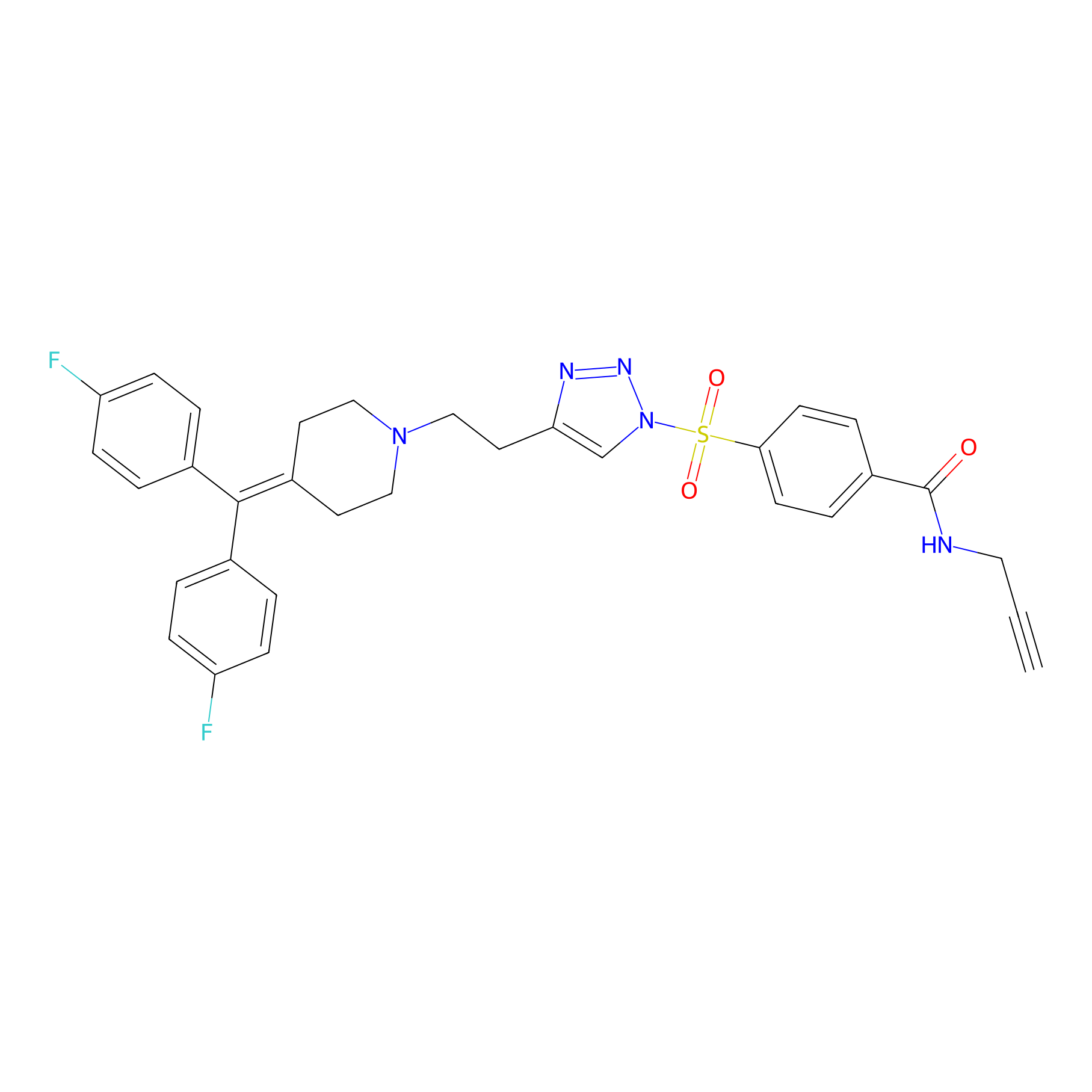 |
Y65(16.78) | LDD0257 | [4] | |
|
TH216 Probe Info |
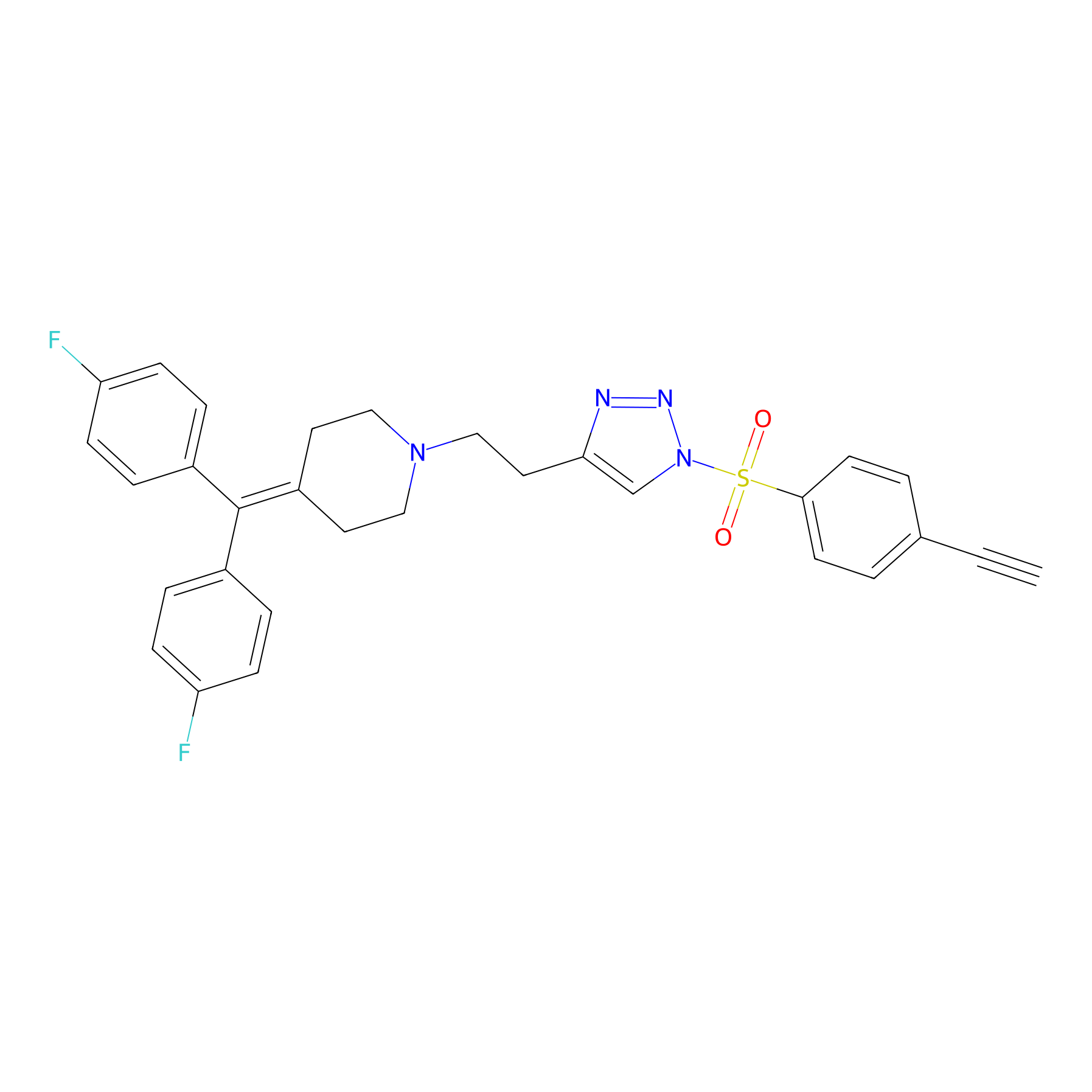 |
Y109(12.60); Y65(11.28) | LDD0259 | [4] | |
|
YN-1 Probe Info |
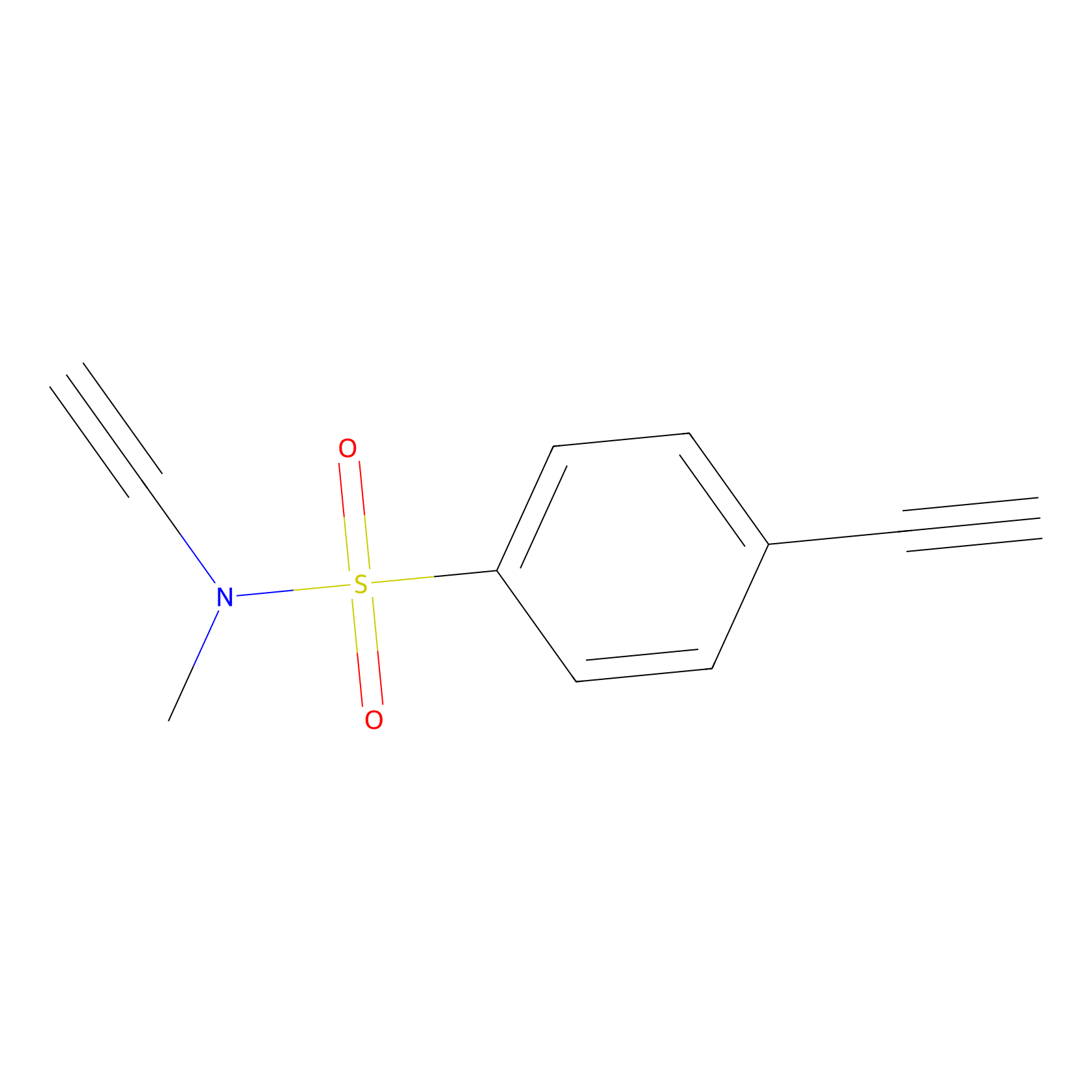 |
100.00 | LDD0444 | [5] | |
|
BTD Probe Info |
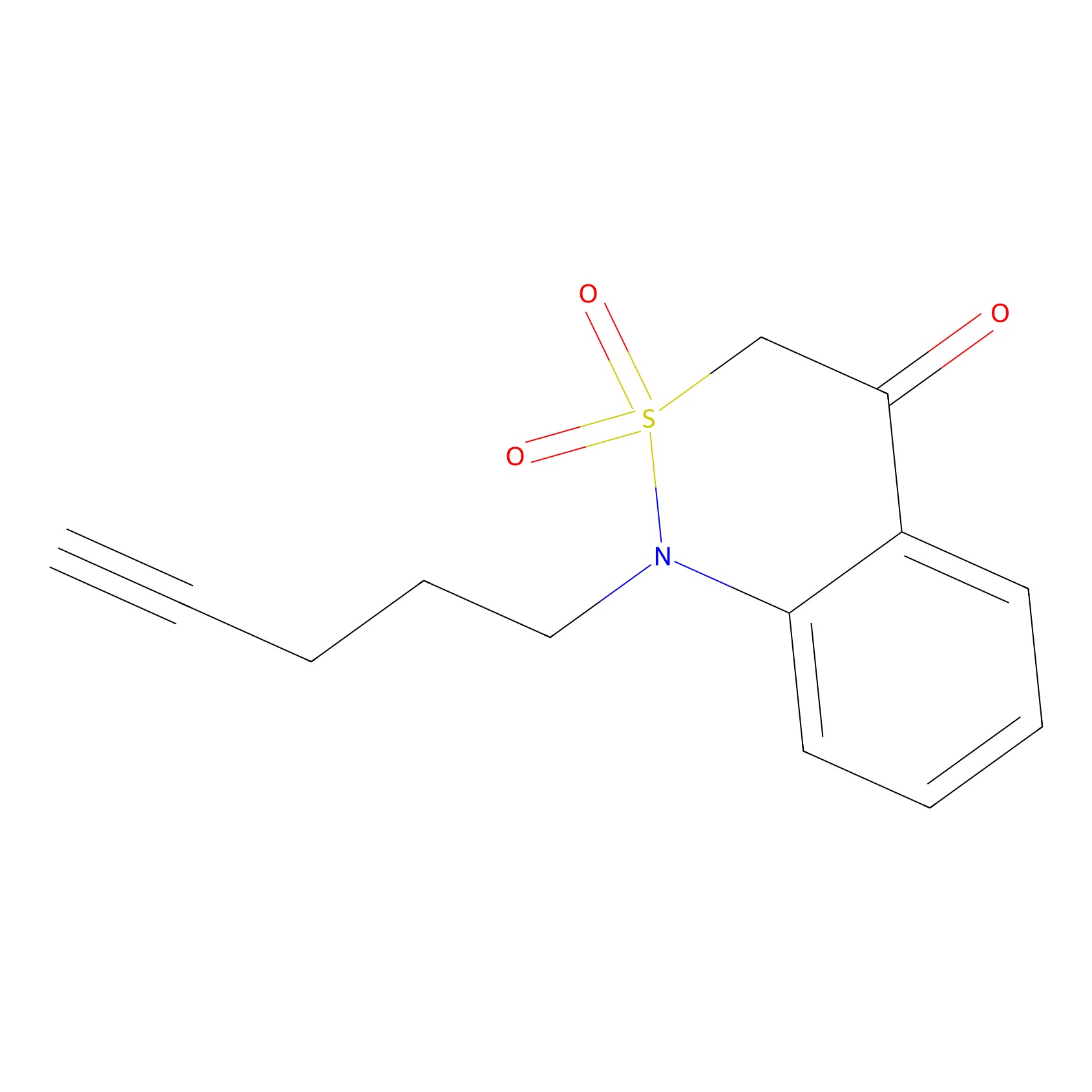 |
C59(4.20) | LDD1699 | [6] | |
|
AZ-9 Probe Info |
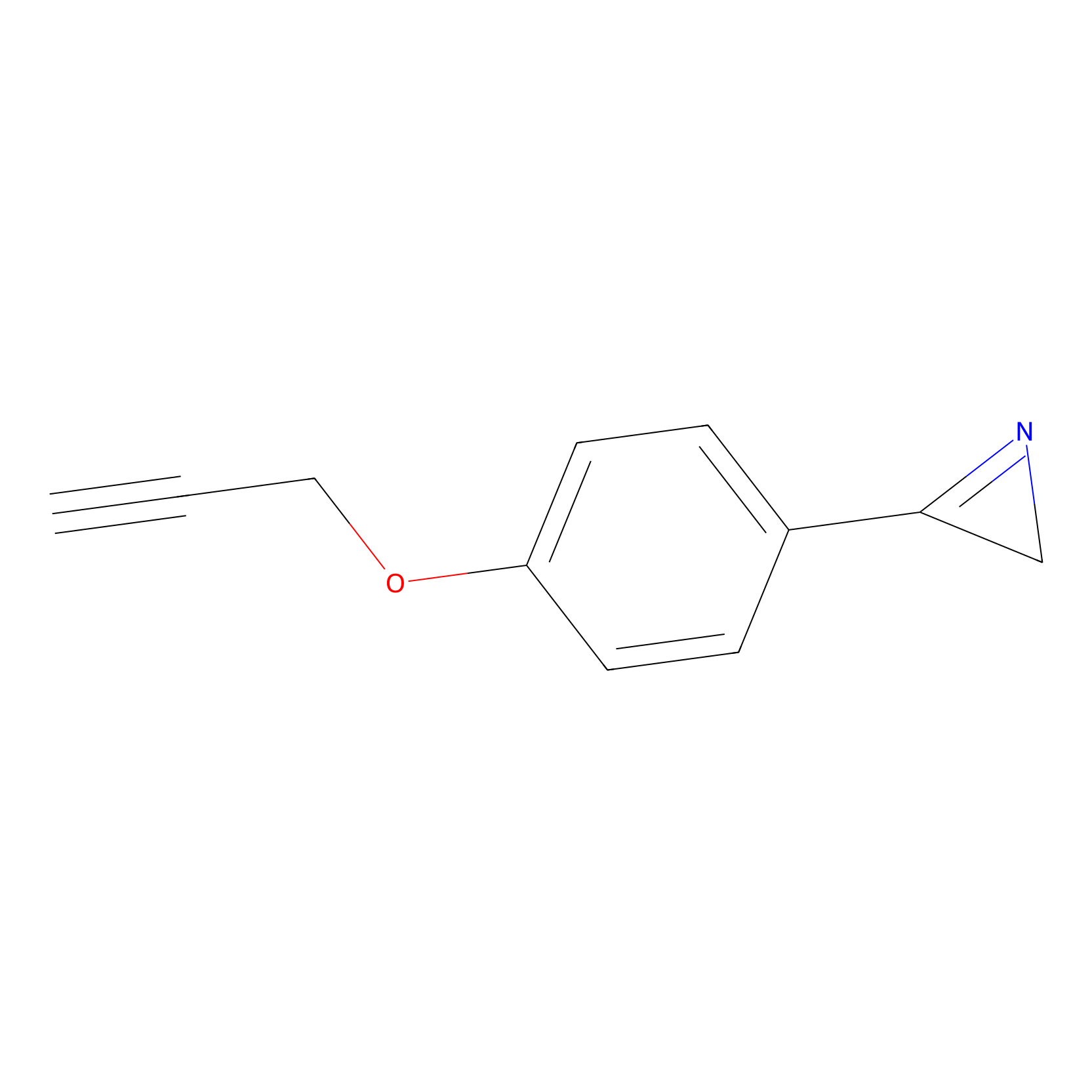 |
E90(0.98) | LDD2208 | [7] | |
|
ONAyne Probe Info |
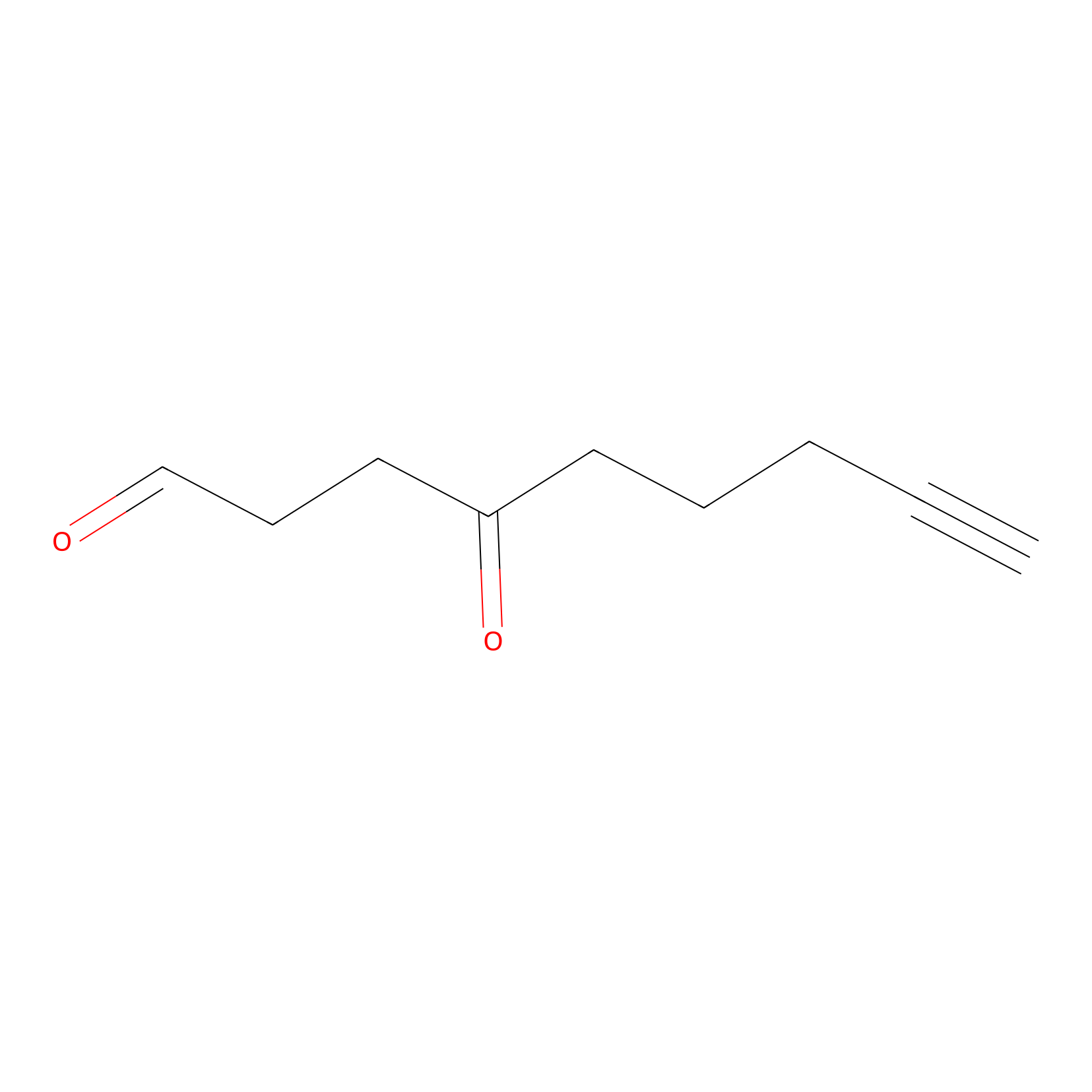 |
K66(0.00); K114(0.00) | LDD0273 | [8] | |
|
OPA-S-S-alkyne Probe Info |
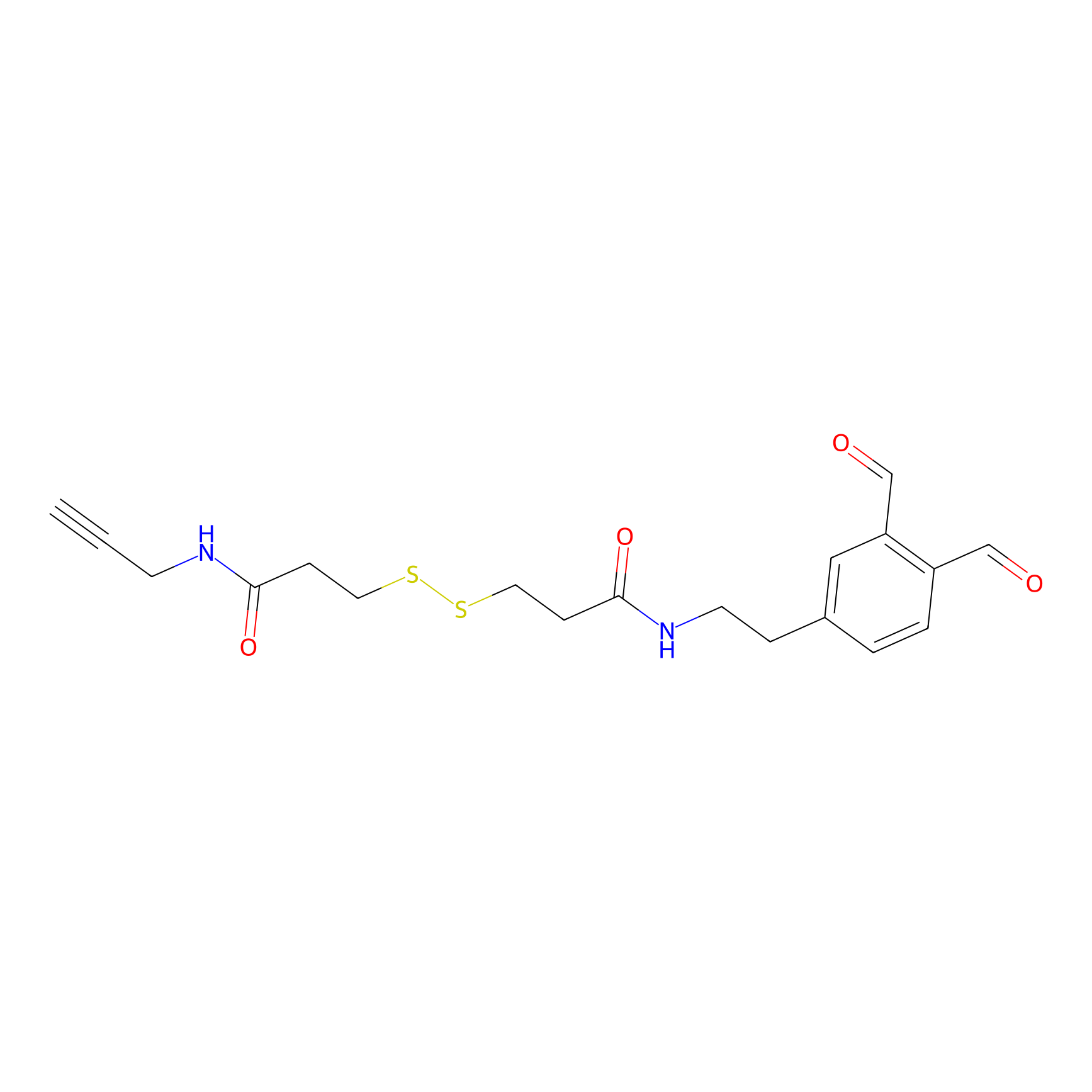 |
K114(2.69); K66(2.69) | LDD3494 | [9] | |
|
AMP probe Probe Info |
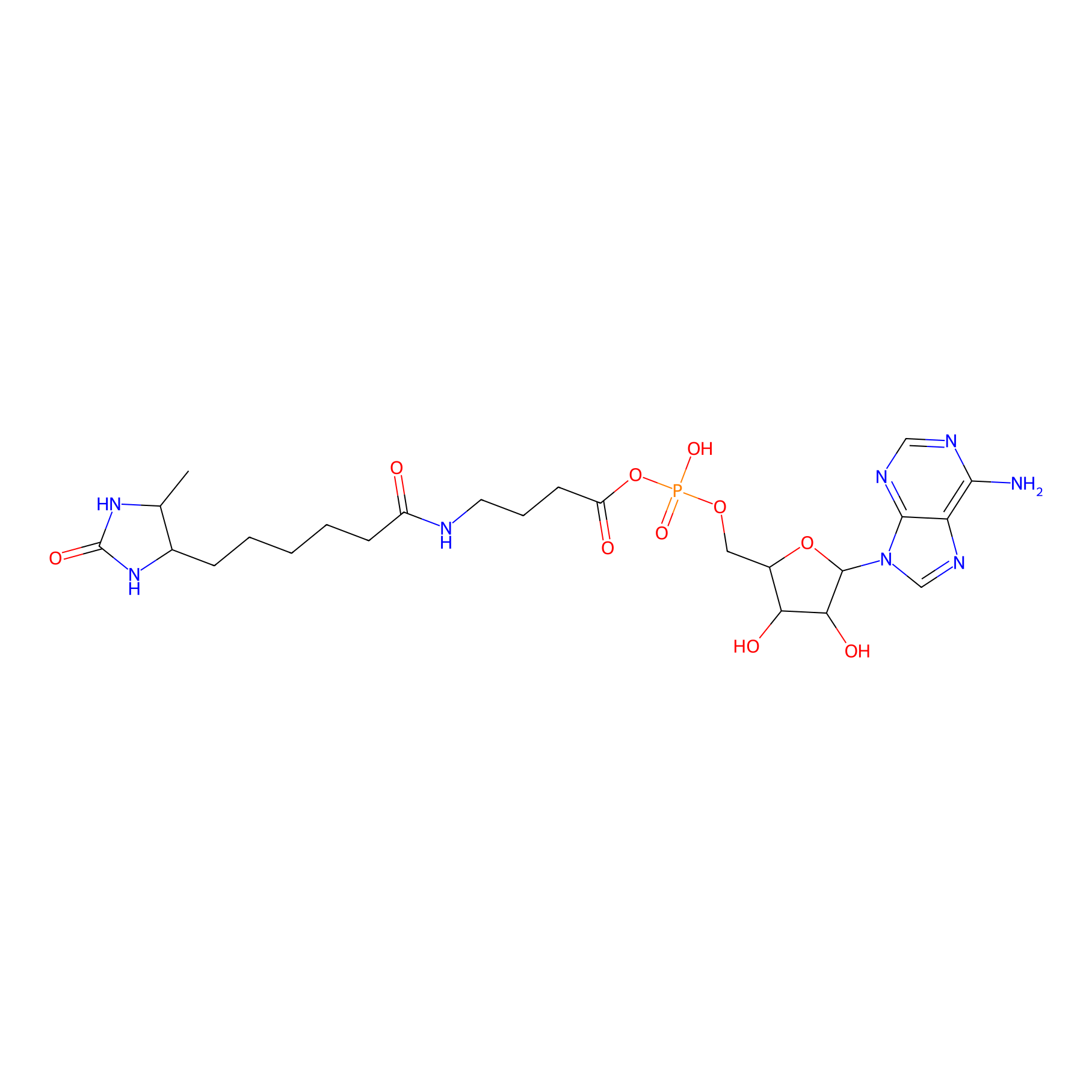 |
K43(0.00); K52(0.00); K94(0.00) | LDD0200 | [10] | |
|
ATP probe Probe Info |
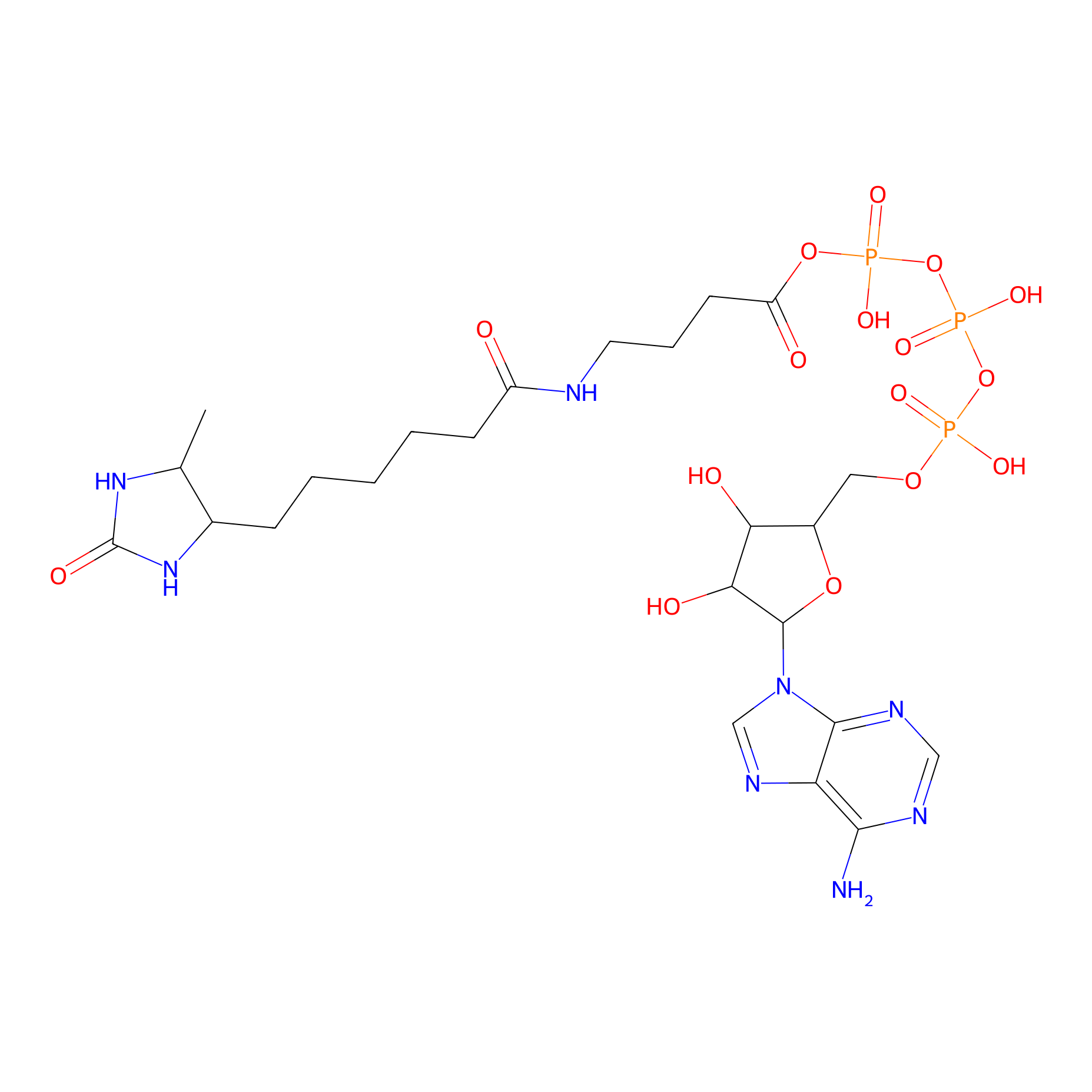 |
K43(0.00); K52(0.00); K94(0.00); K66(0.00) | LDD0199 | [10] | |
|
4-Iodoacetamidophenylacetylene Probe Info |
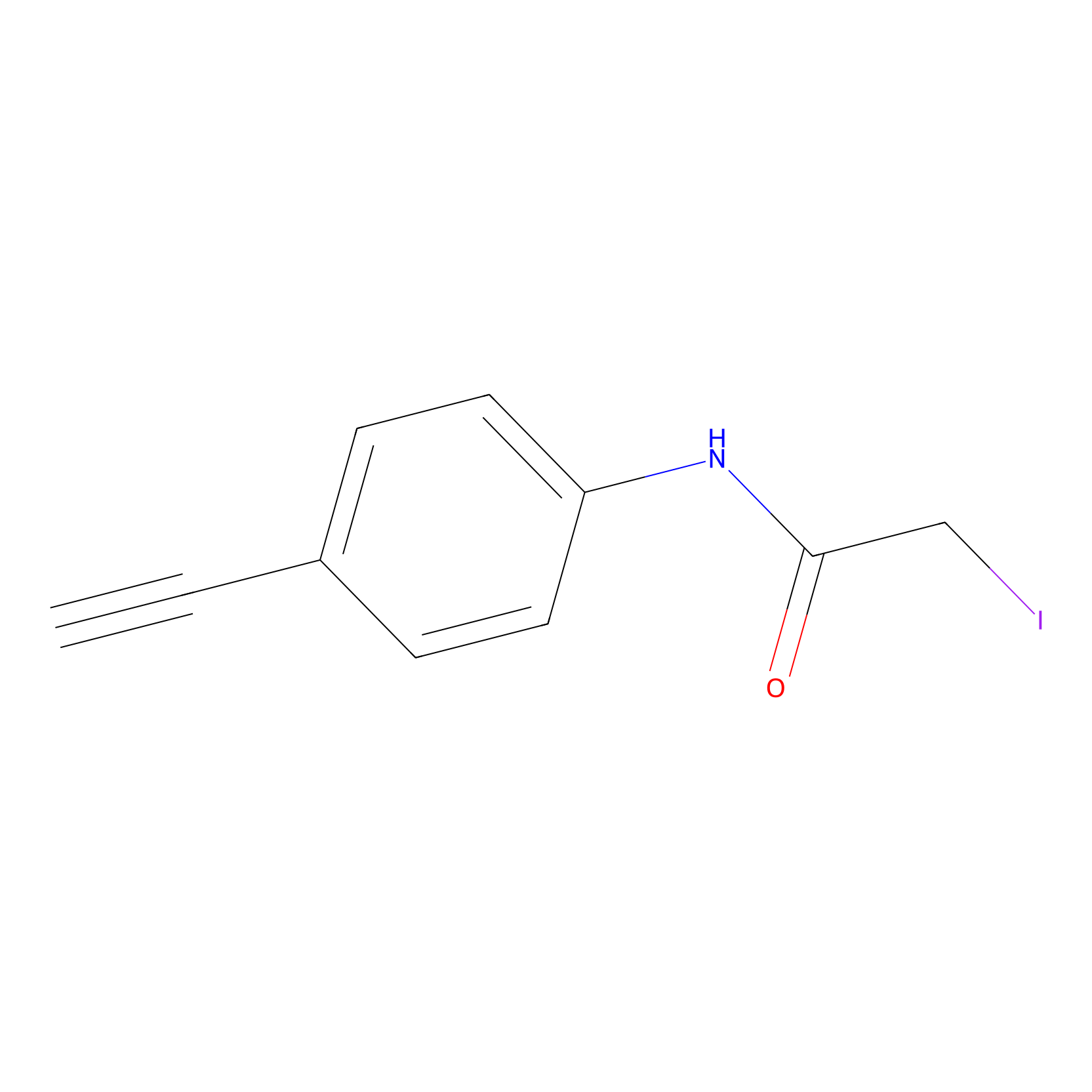 |
N.A. | LDD0038 | [11] | |
|
IA-alkyne Probe Info |
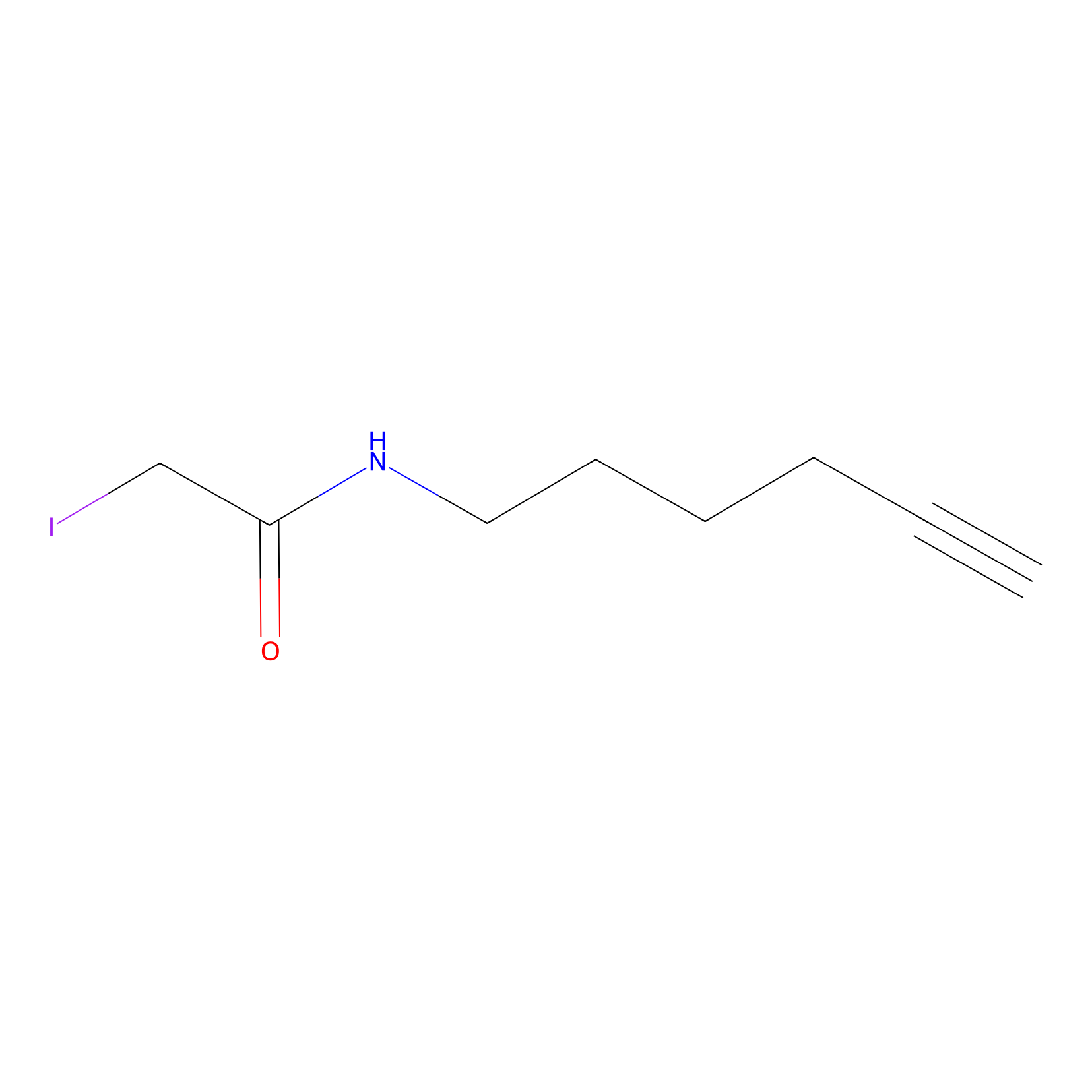 |
N.A. | LDD0032 | [12] | |
|
Lodoacetamide azide Probe Info |
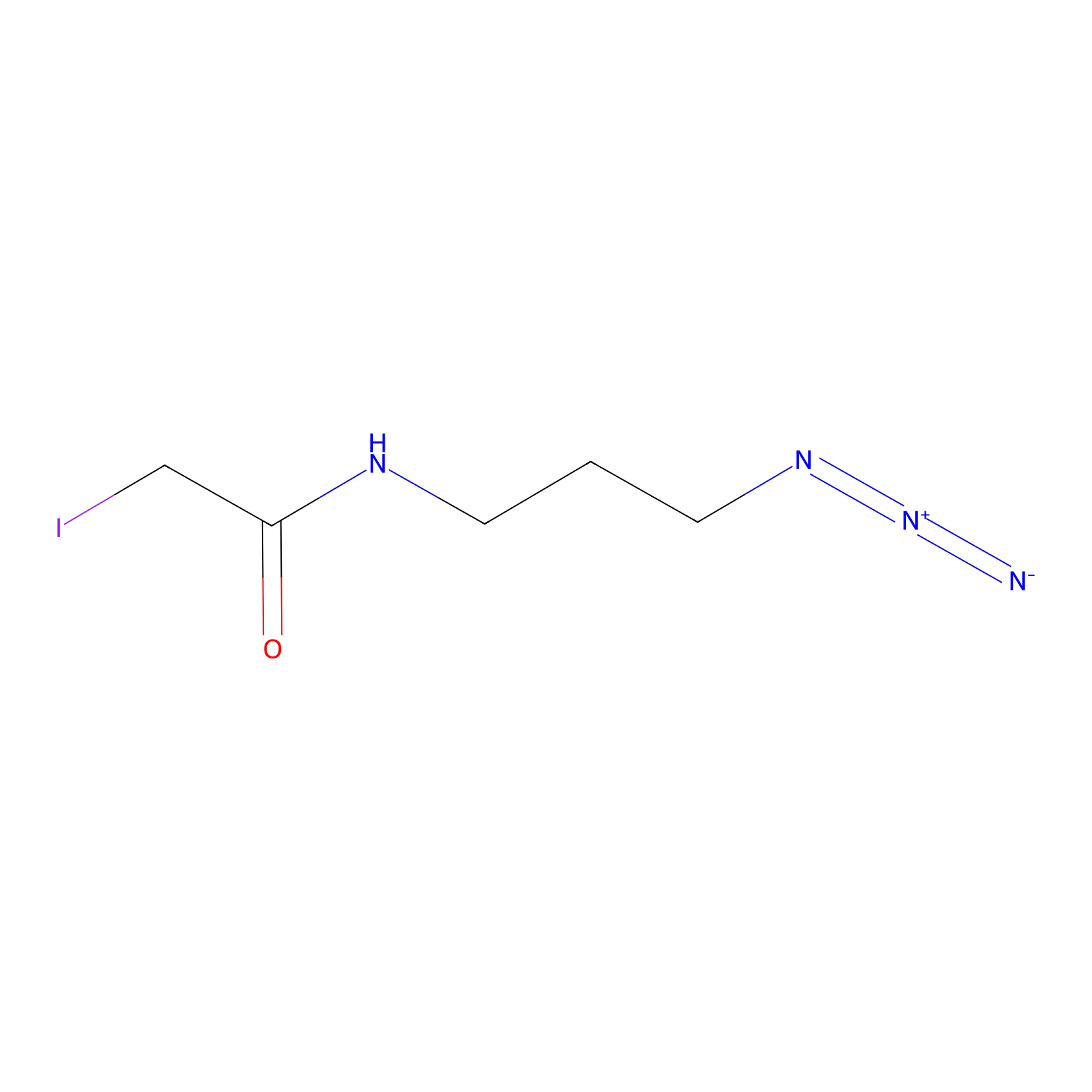 |
N.A. | LDD0037 | [11] | |
|
ATP probe Probe Info |
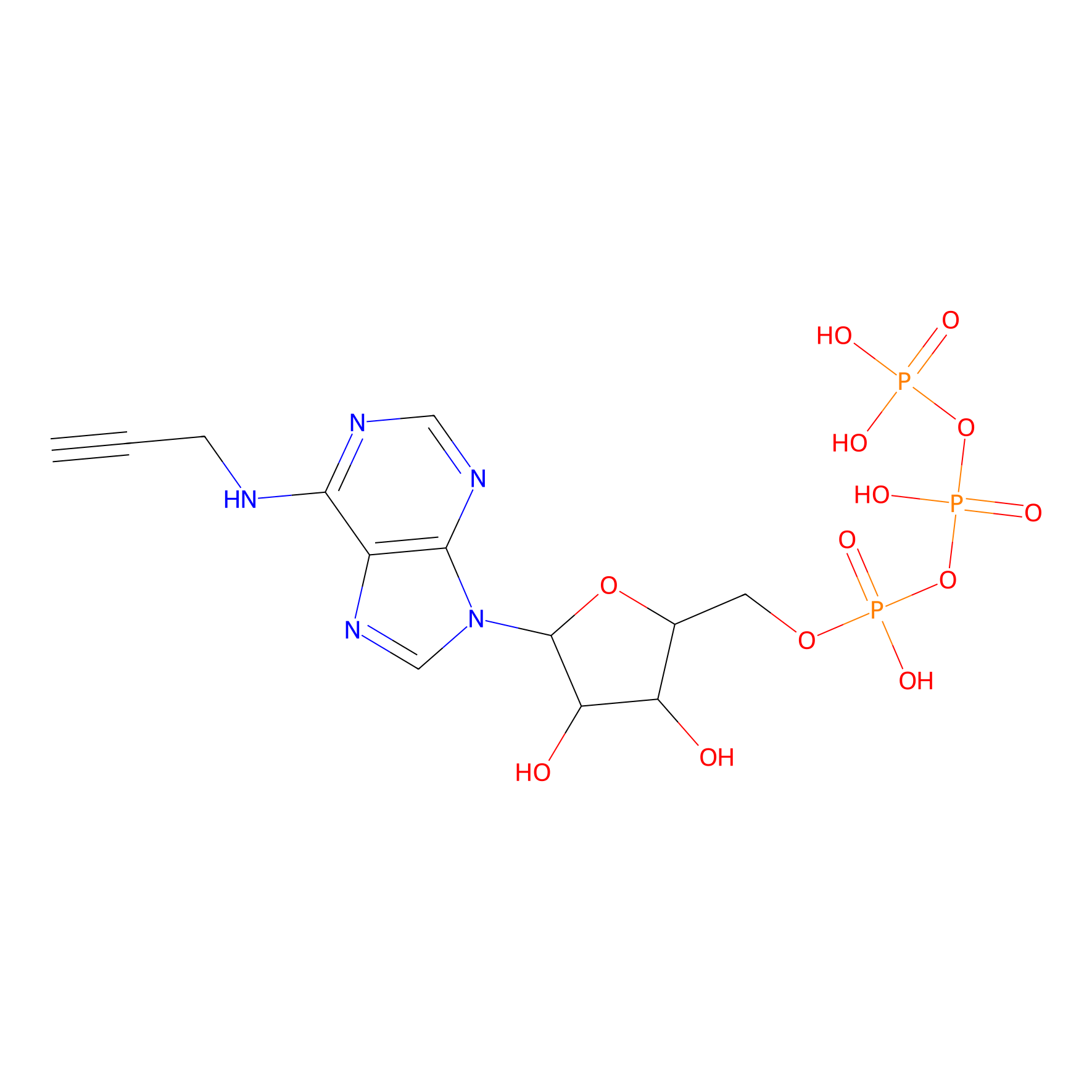 |
K114(0.00); K94(0.00) | LDD0035 | [13] | |
|
IPM Probe Info |
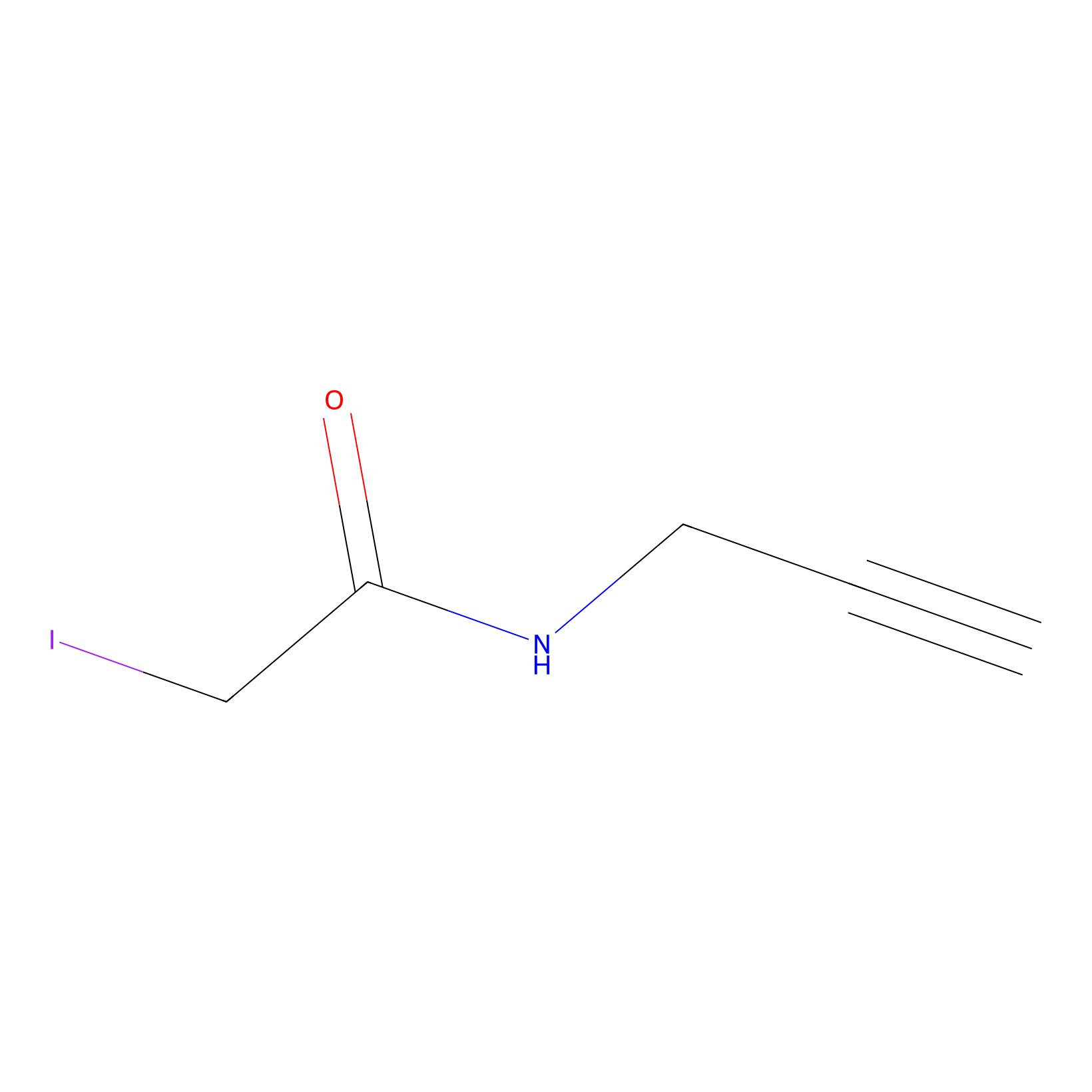 |
N.A. | LDD0025 | [14] | |
|
JW-RF-010 Probe Info |
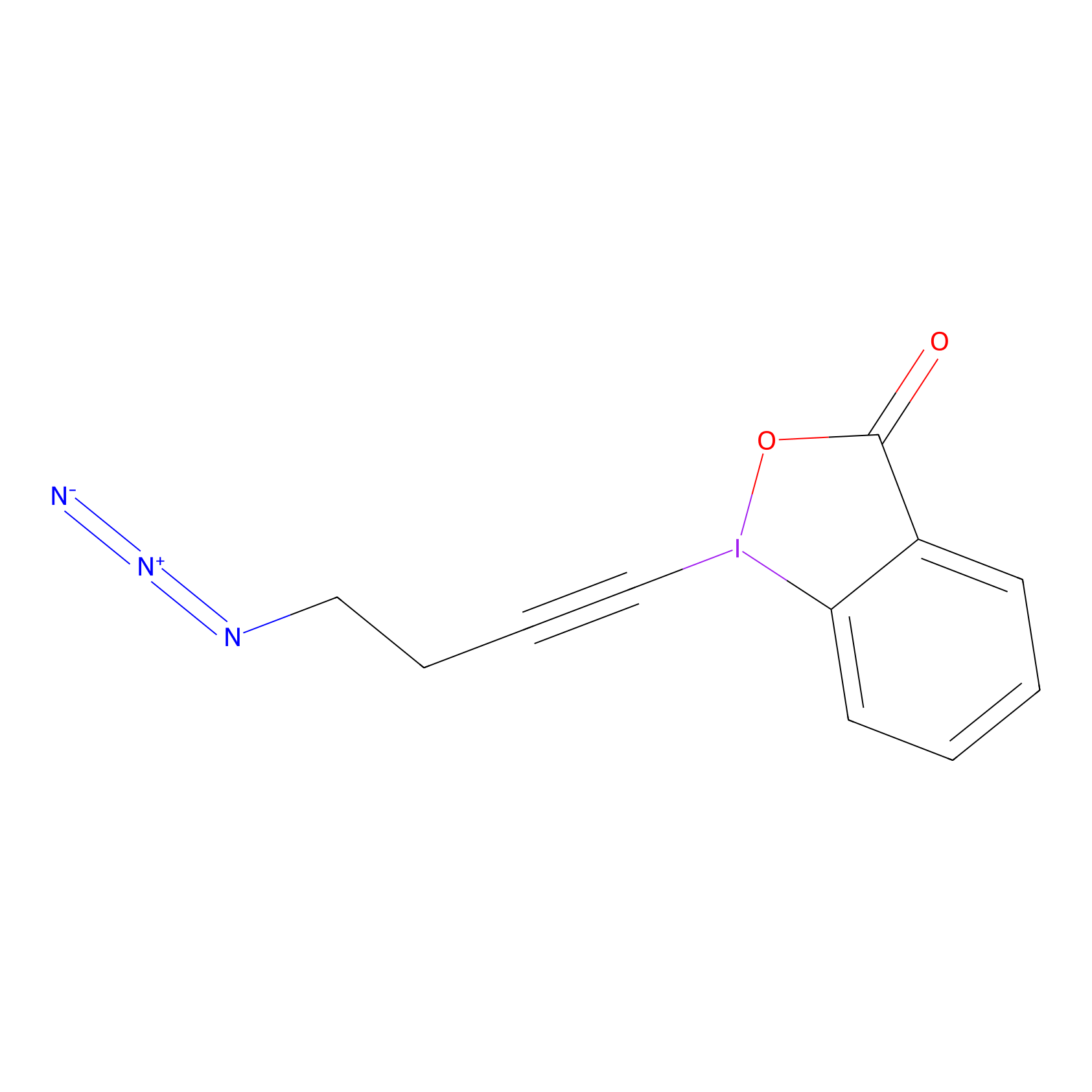 |
N.A. | LDD0026 | [14] | |
|
NAIA_4 Probe Info |
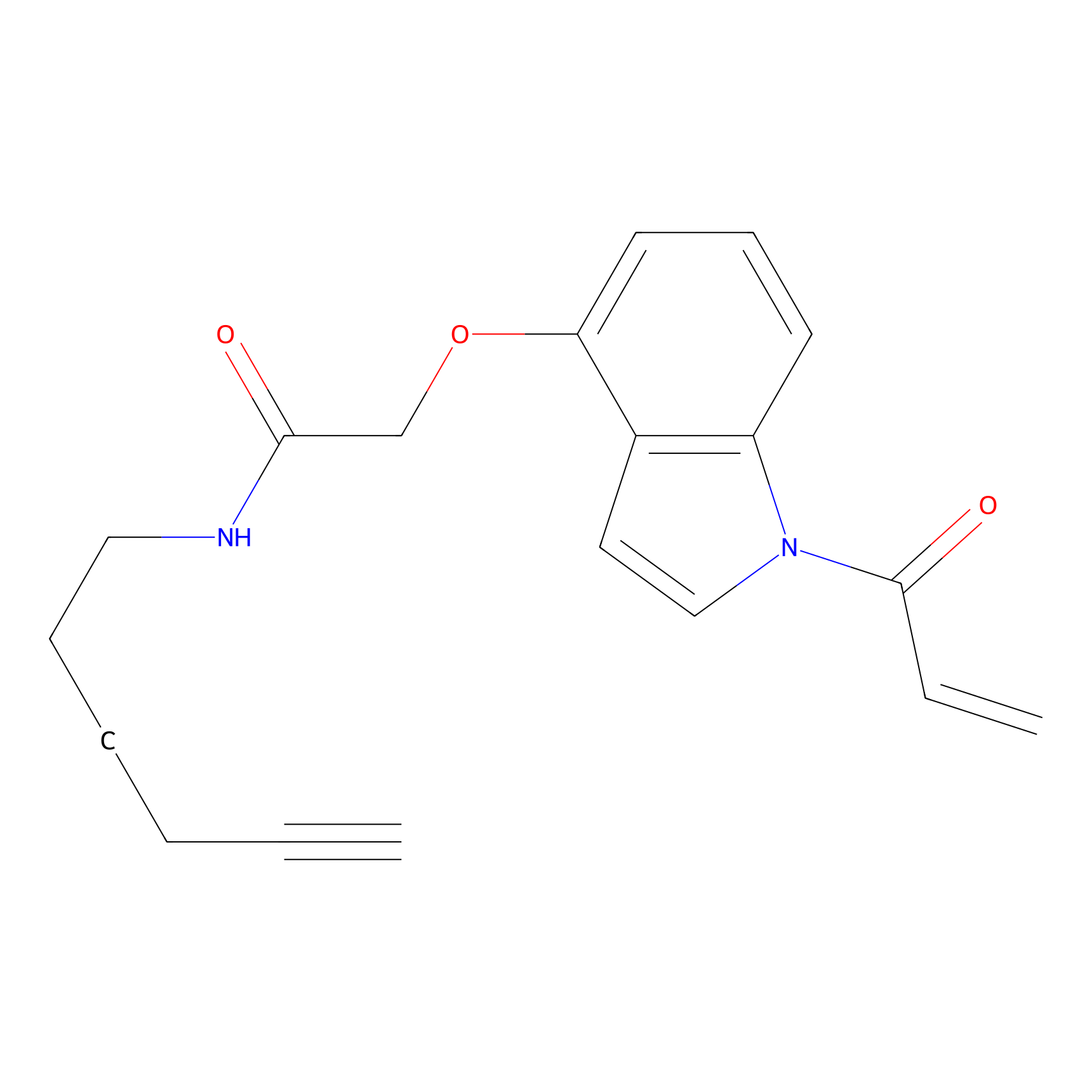 |
N.A. | LDD2226 | [15] | |
|
TFBX Probe Info |
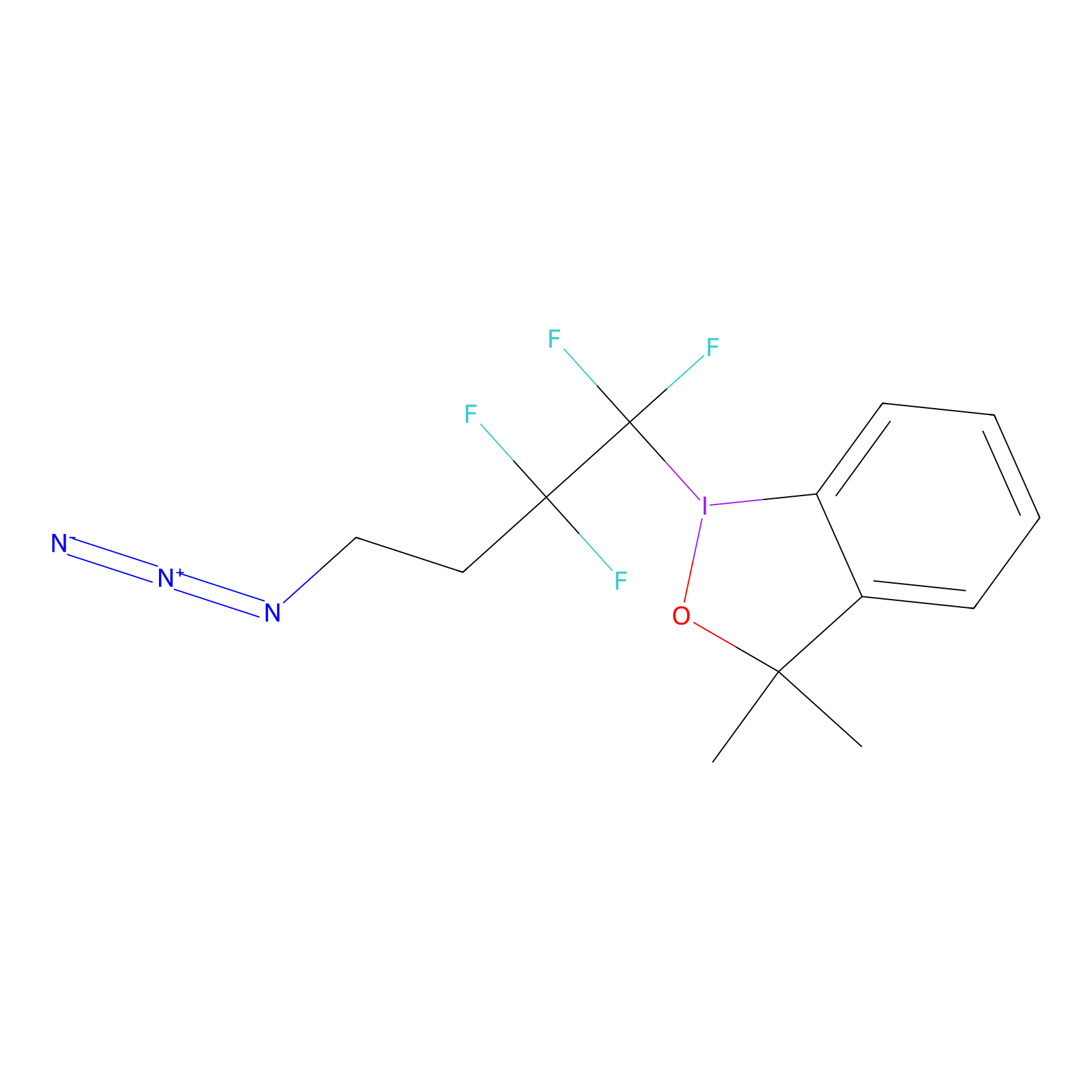 |
N.A. | LDD0027 | [14] | |
|
Compound 10 Probe Info |
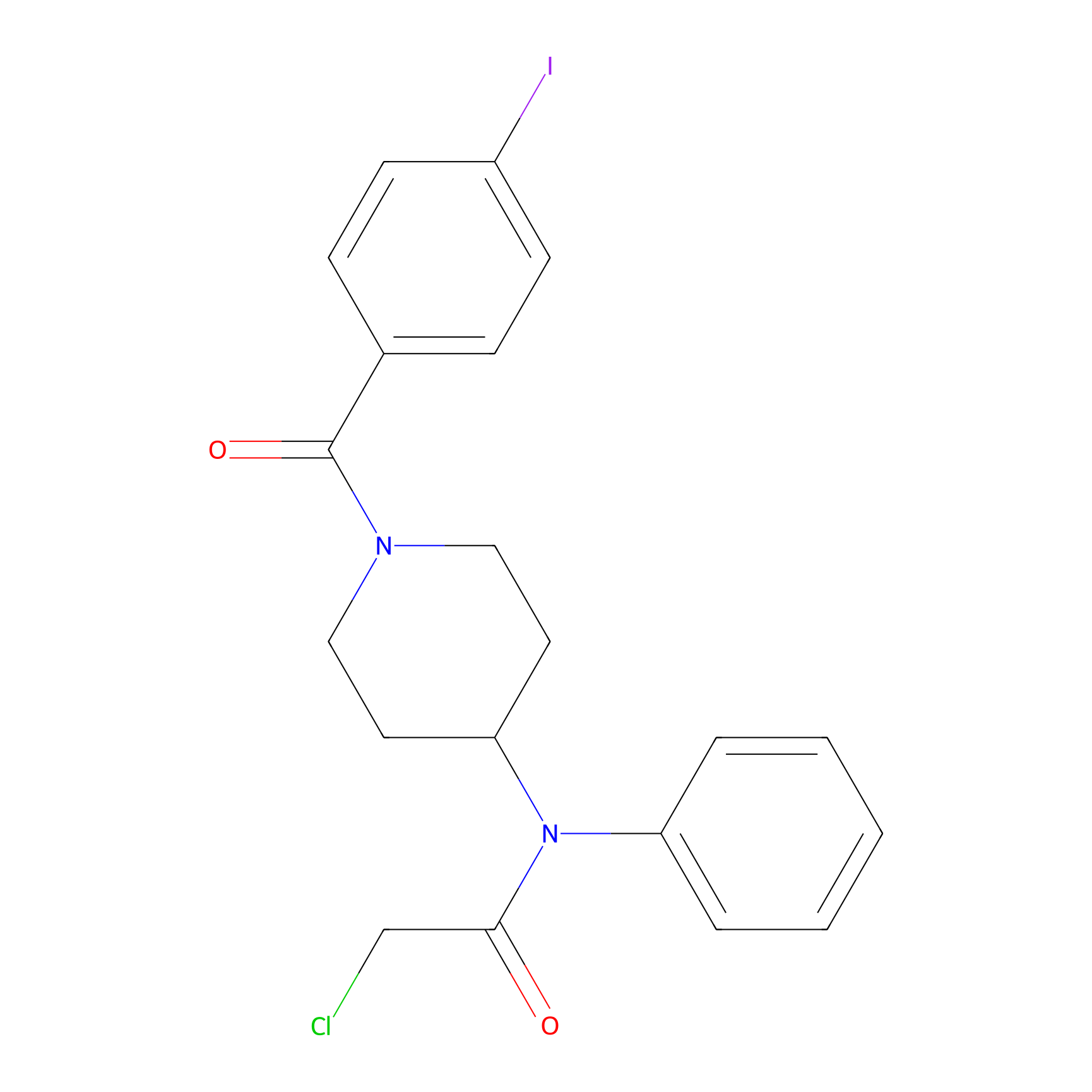 |
N.A. | LDD2216 | [16] | |
|
DBIA Probe Info |
 |
C59(0.18) | LDD2236 | [17] | |
|
NHS Probe Info |
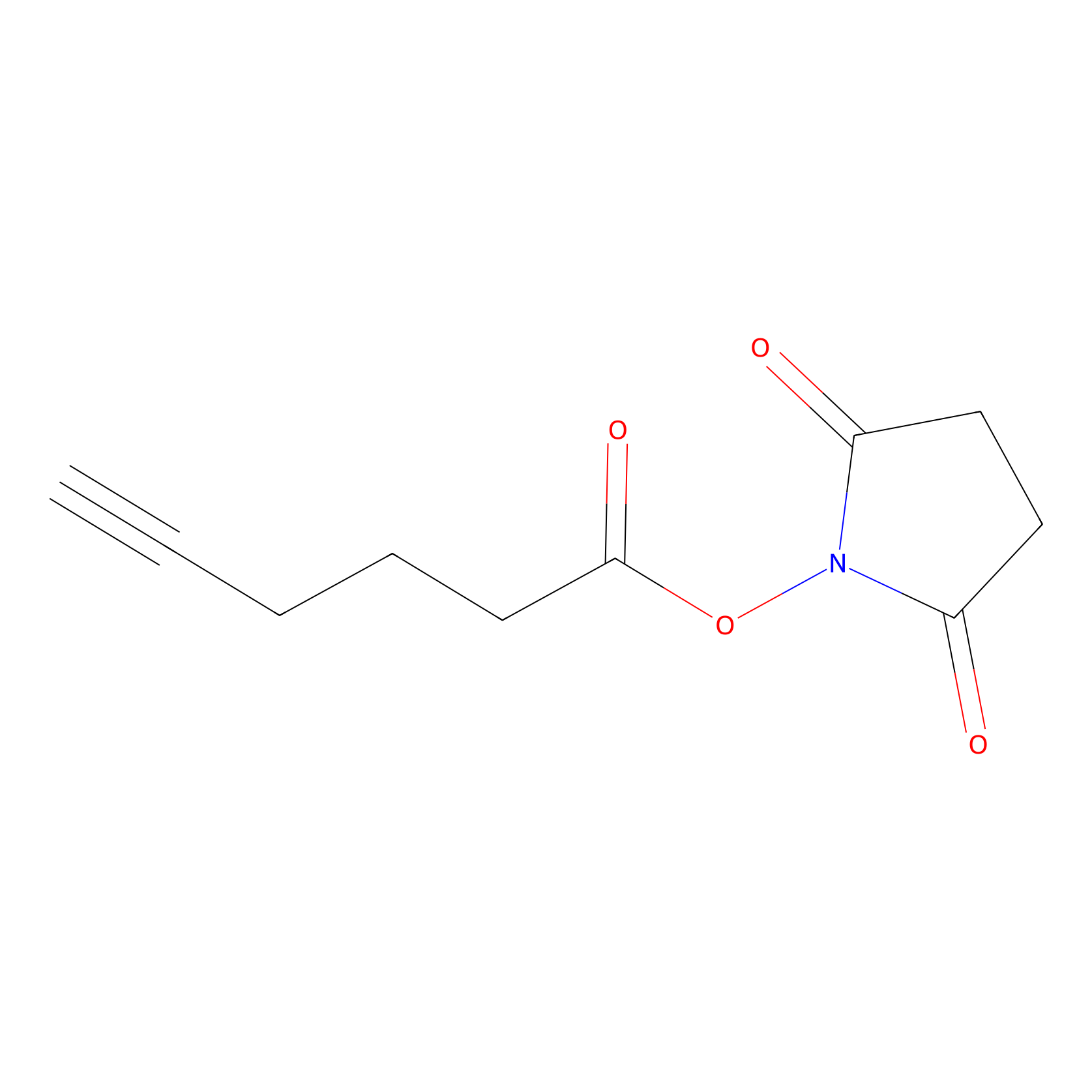 |
K66(0.00); K52(0.00) | LDD0010 | [18] | |
|
OSF Probe Info |
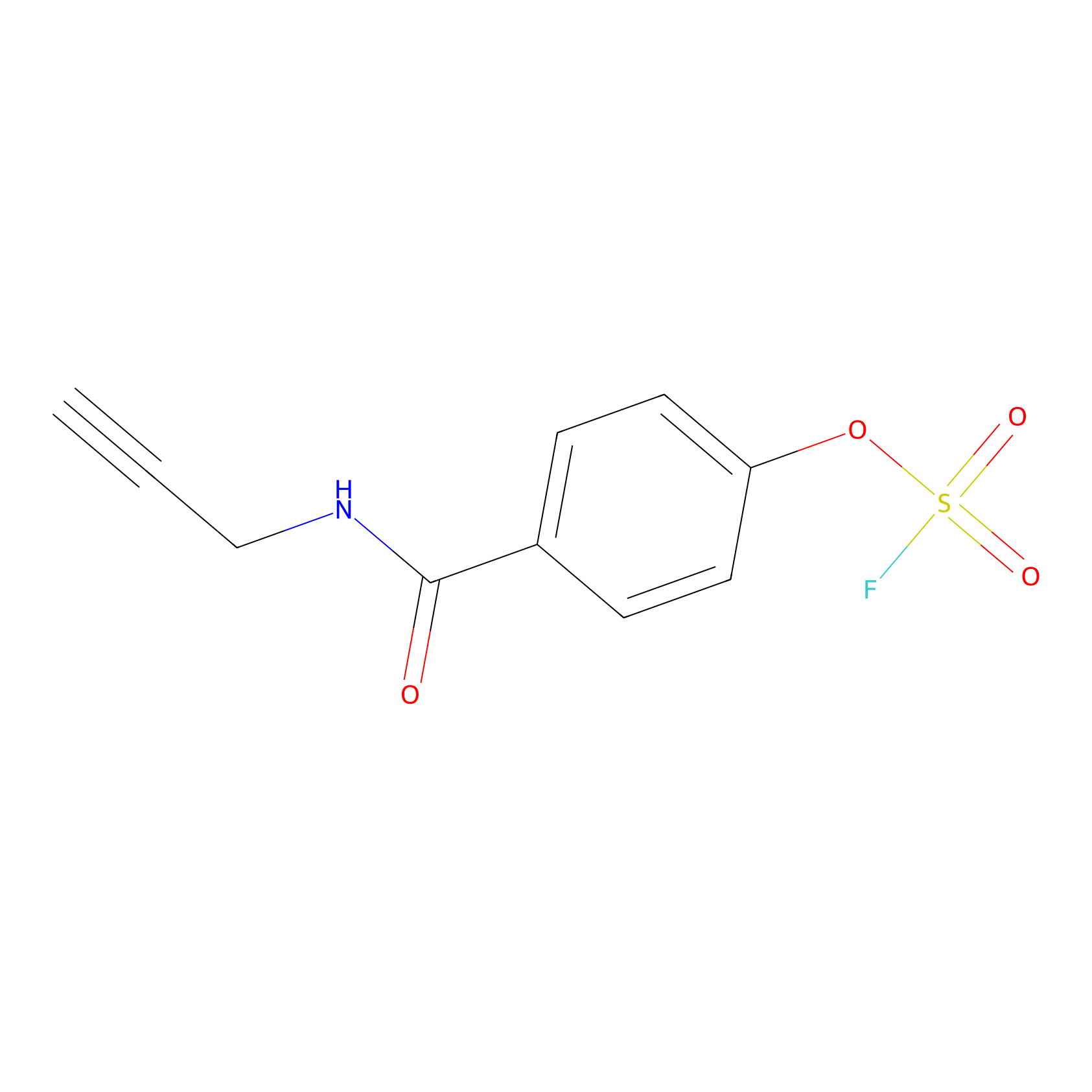 |
N.A. | LDD0029 | [19] | |
|
SF Probe Info |
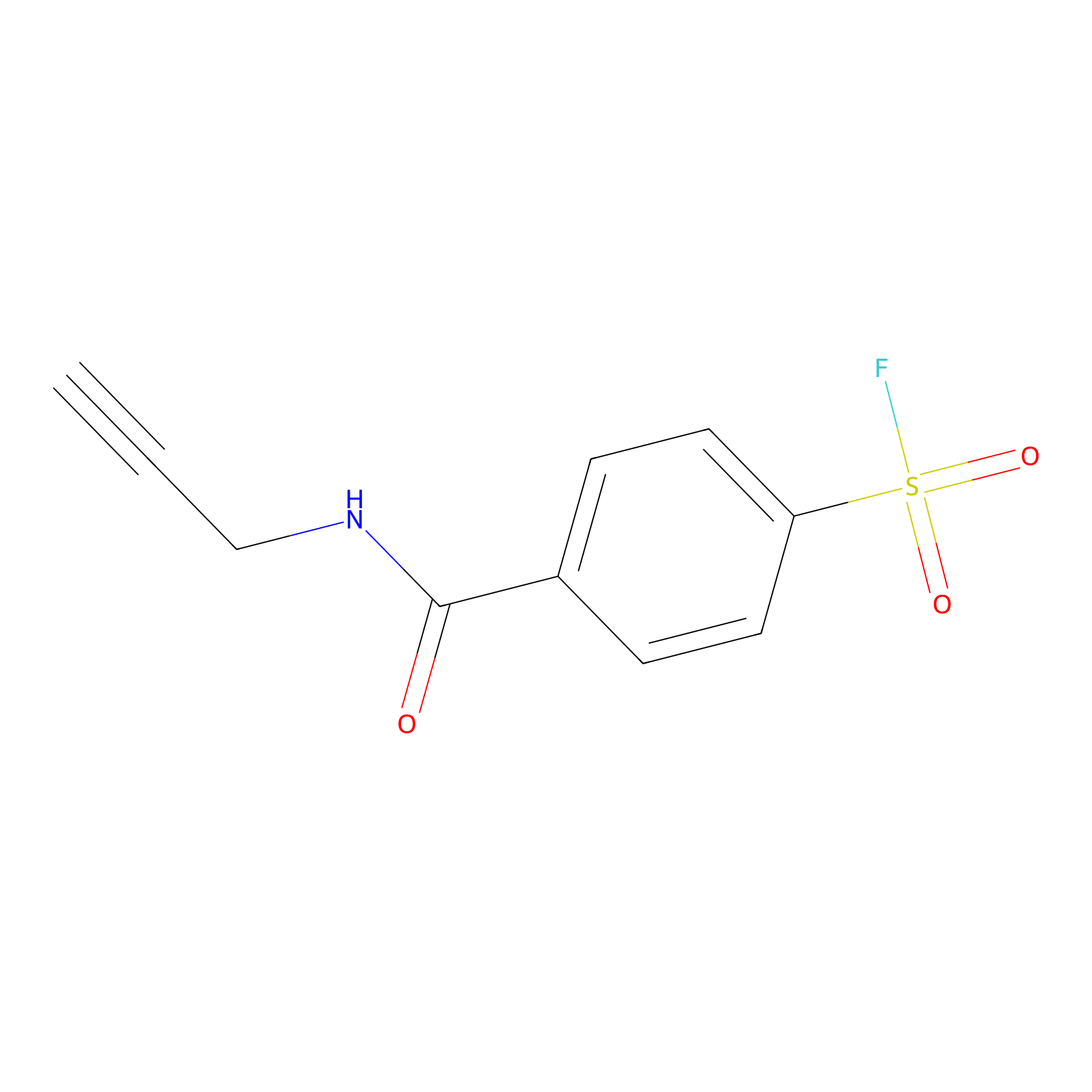 |
K102(0.00); Y109(0.00); K18(0.00); K94(0.00) | LDD0028 | [19] | |
|
STPyne Probe Info |
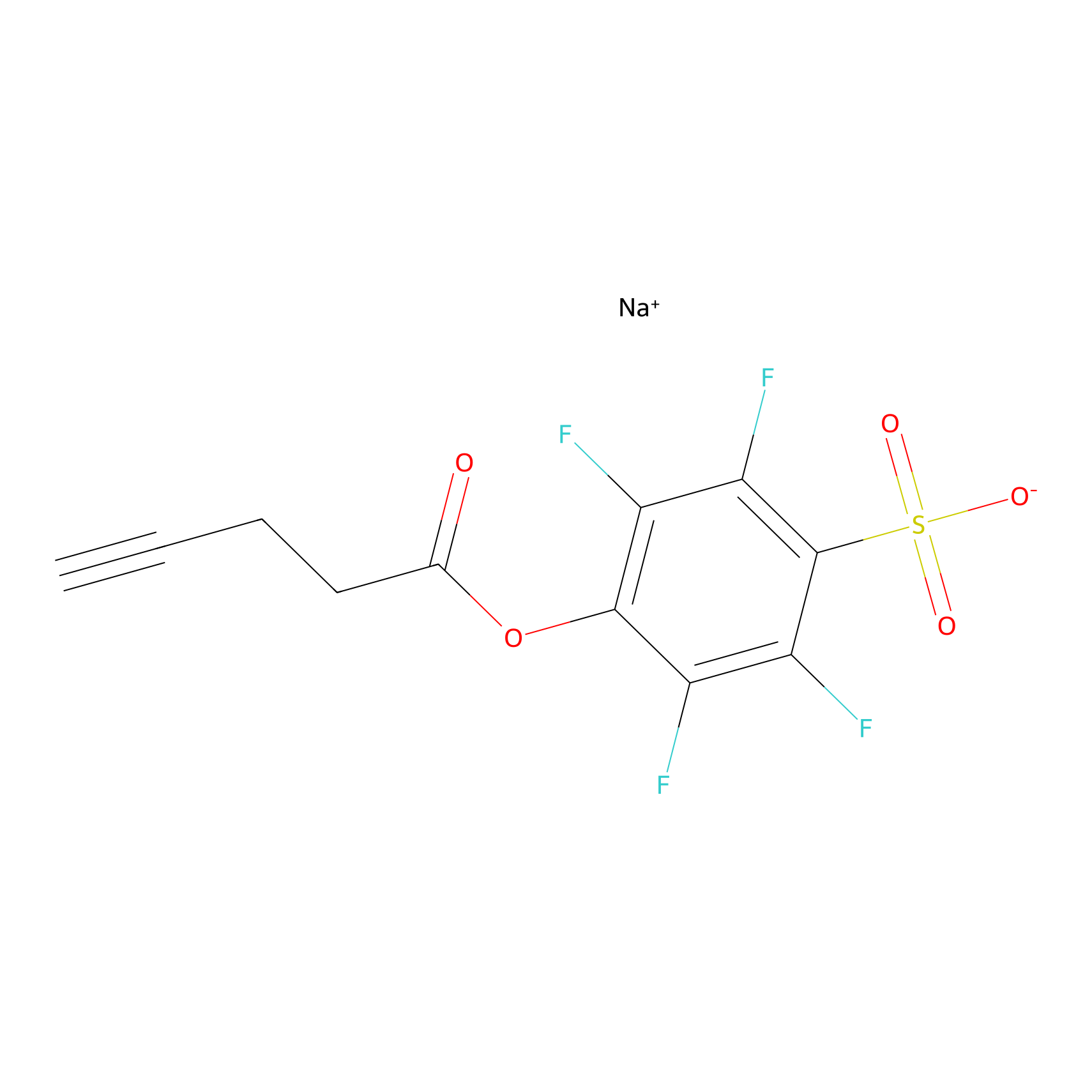 |
K52(0.00); K66(0.00) | LDD0009 | [18] | |
|
Phosphinate-6 Probe Info |
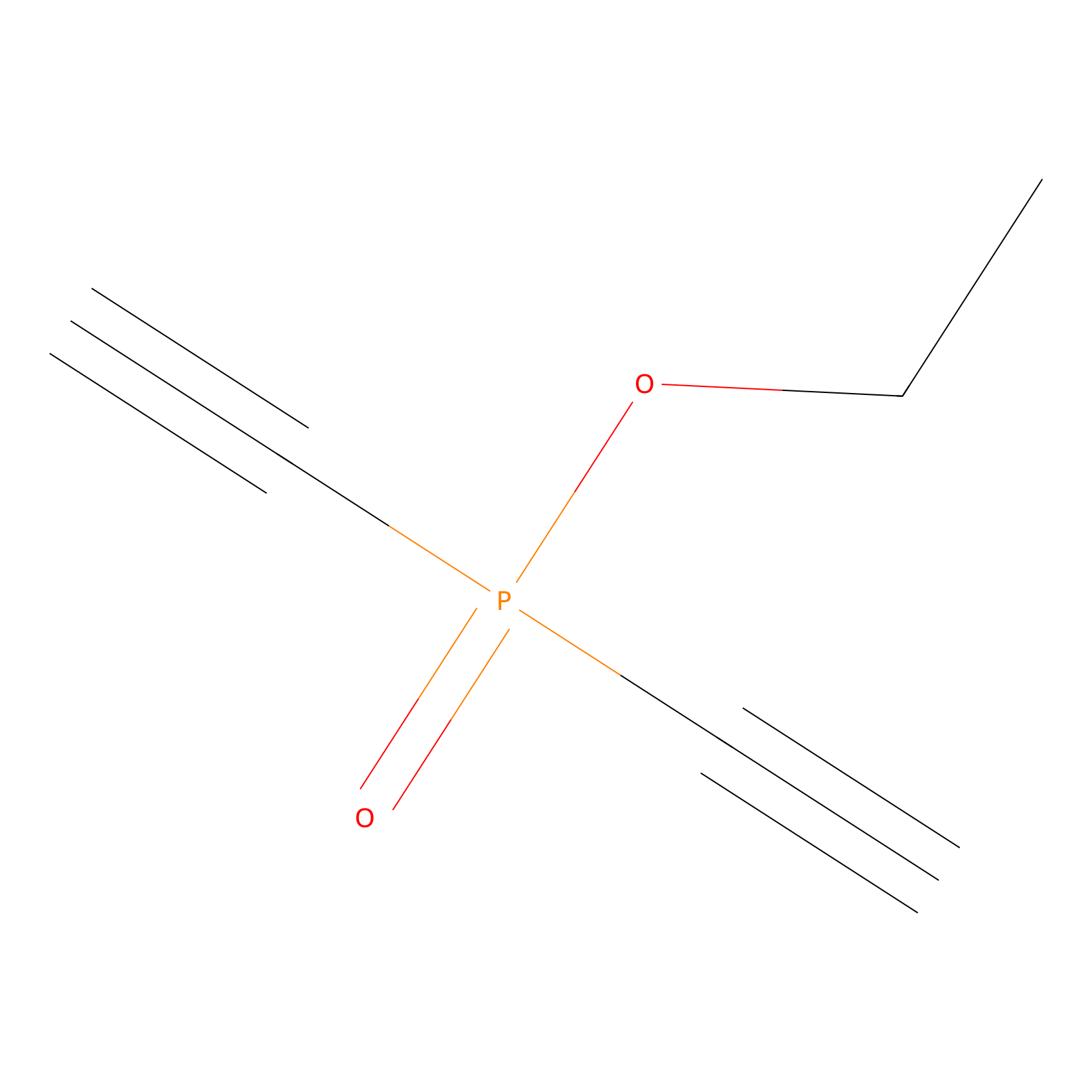 |
N.A. | LDD0018 | [20] | |
|
Ox-W18 Probe Info |
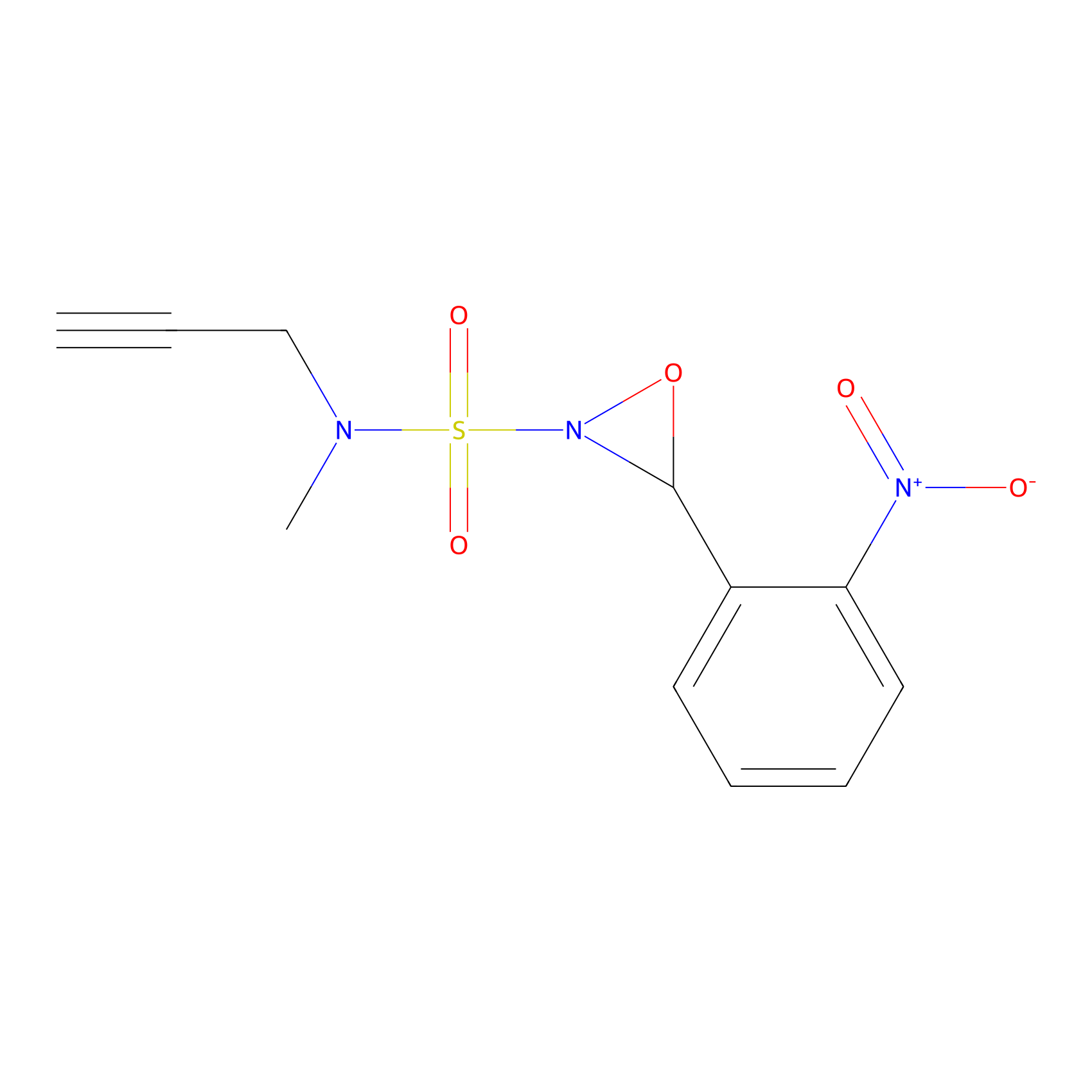 |
N.A. | LDD2175 | [21] | |
|
1c-yne Probe Info |
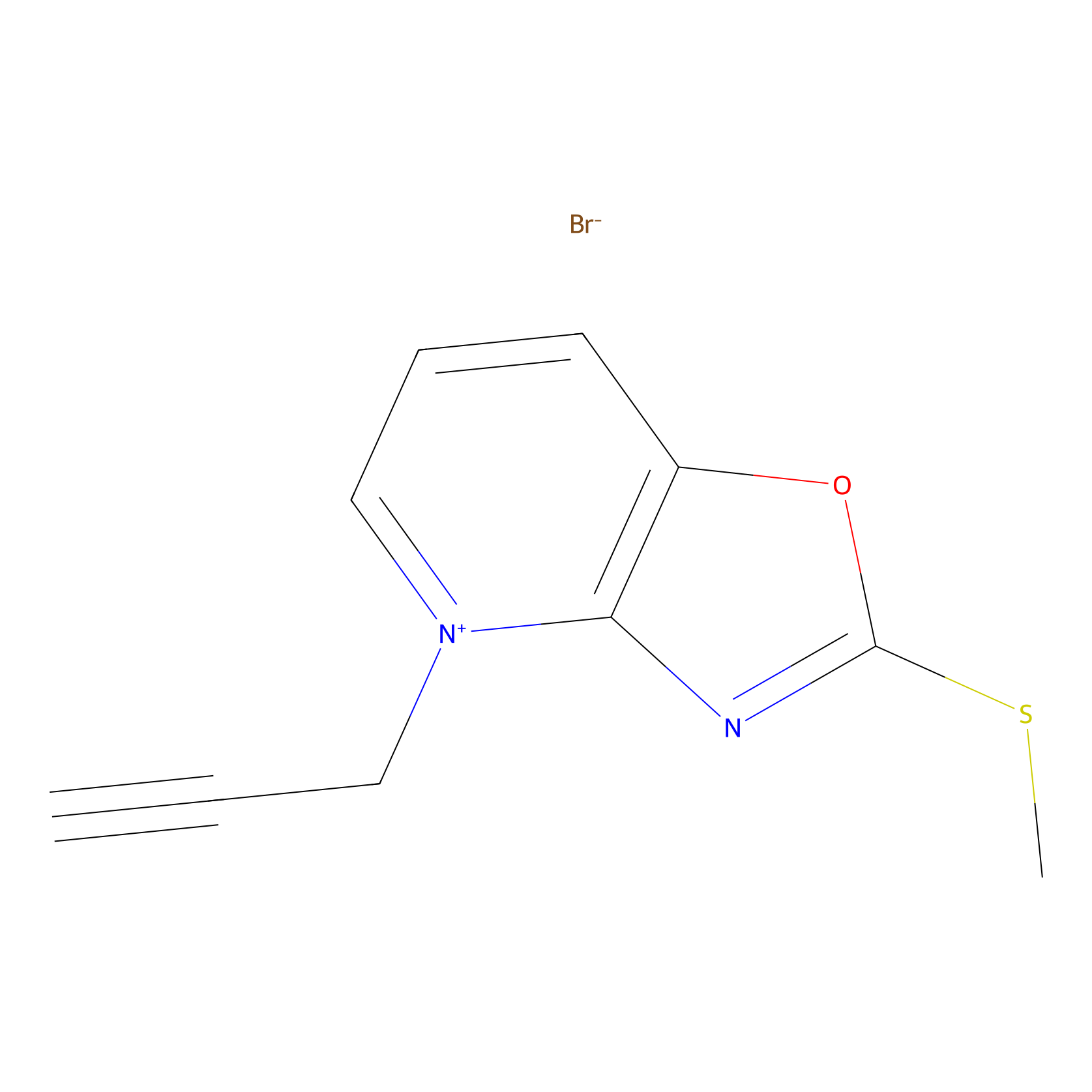 |
K66(0.00); K52(0.00); K43(0.00) | LDD0228 | [22] | |
|
Acrolein Probe Info |
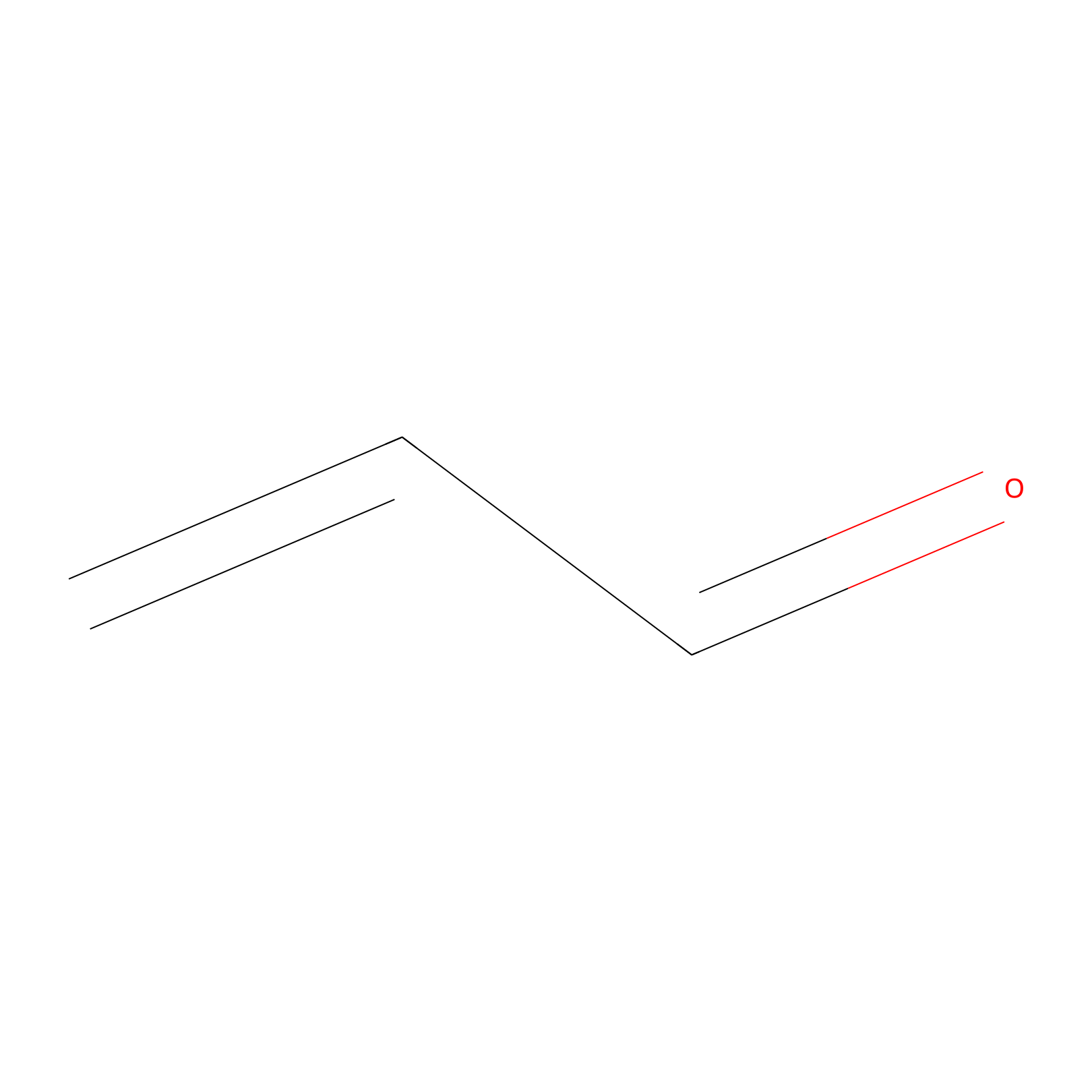 |
N.A. | LDD0217 | [23] | |
|
Methacrolein Probe Info |
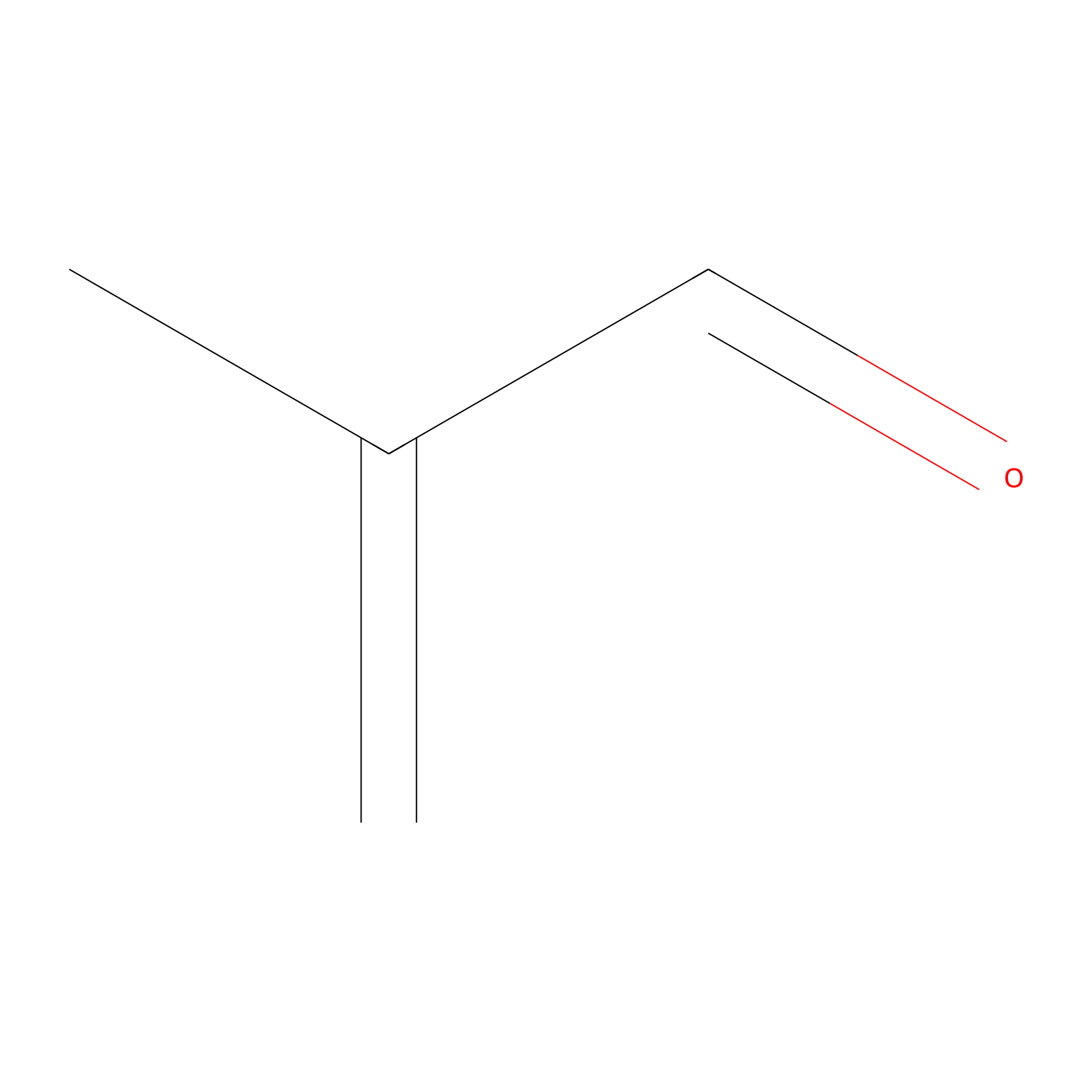 |
N.A. | LDD0218 | [23] | |
|
W1 Probe Info |
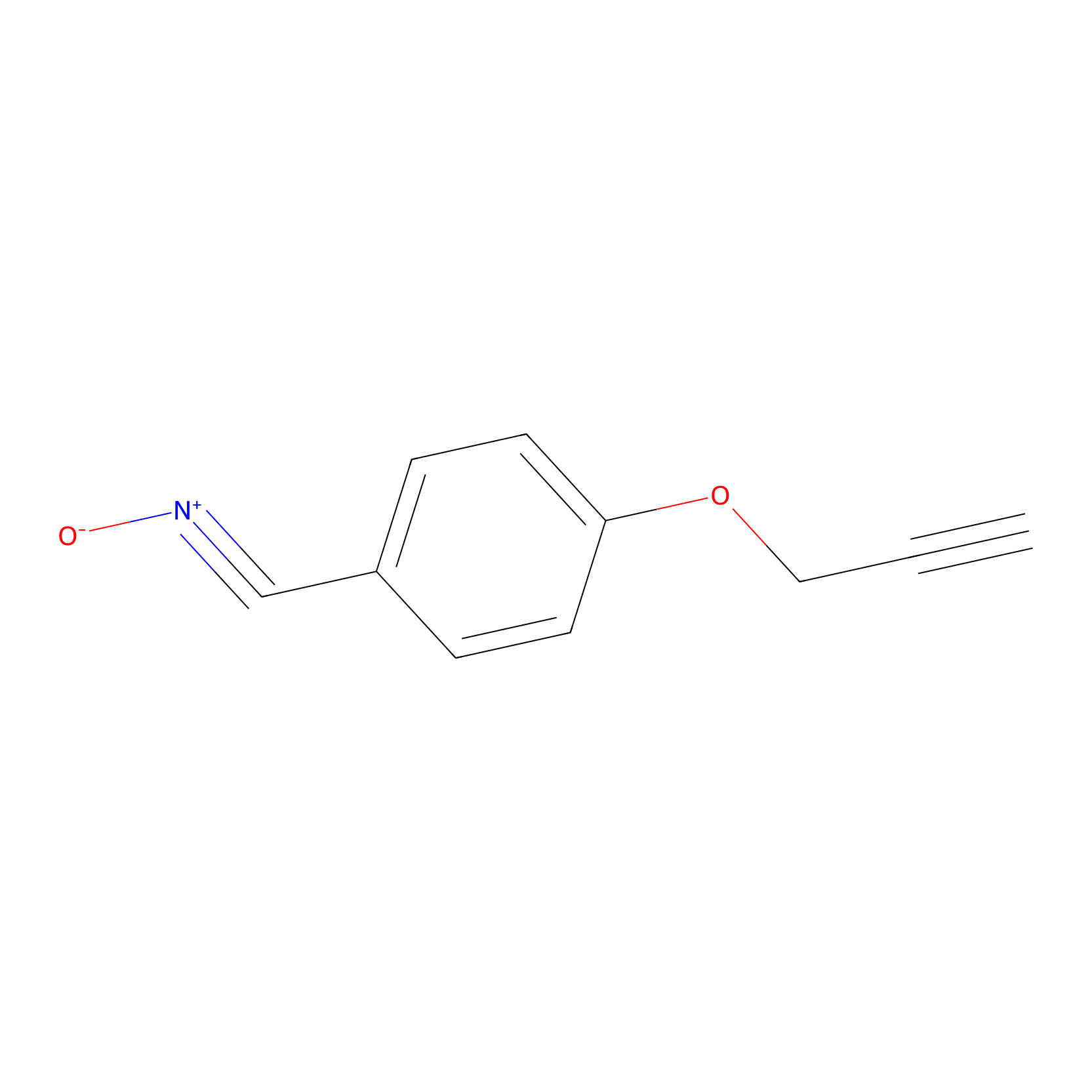 |
N.A. | LDD0236 | [24] | |
|
AOyne Probe Info |
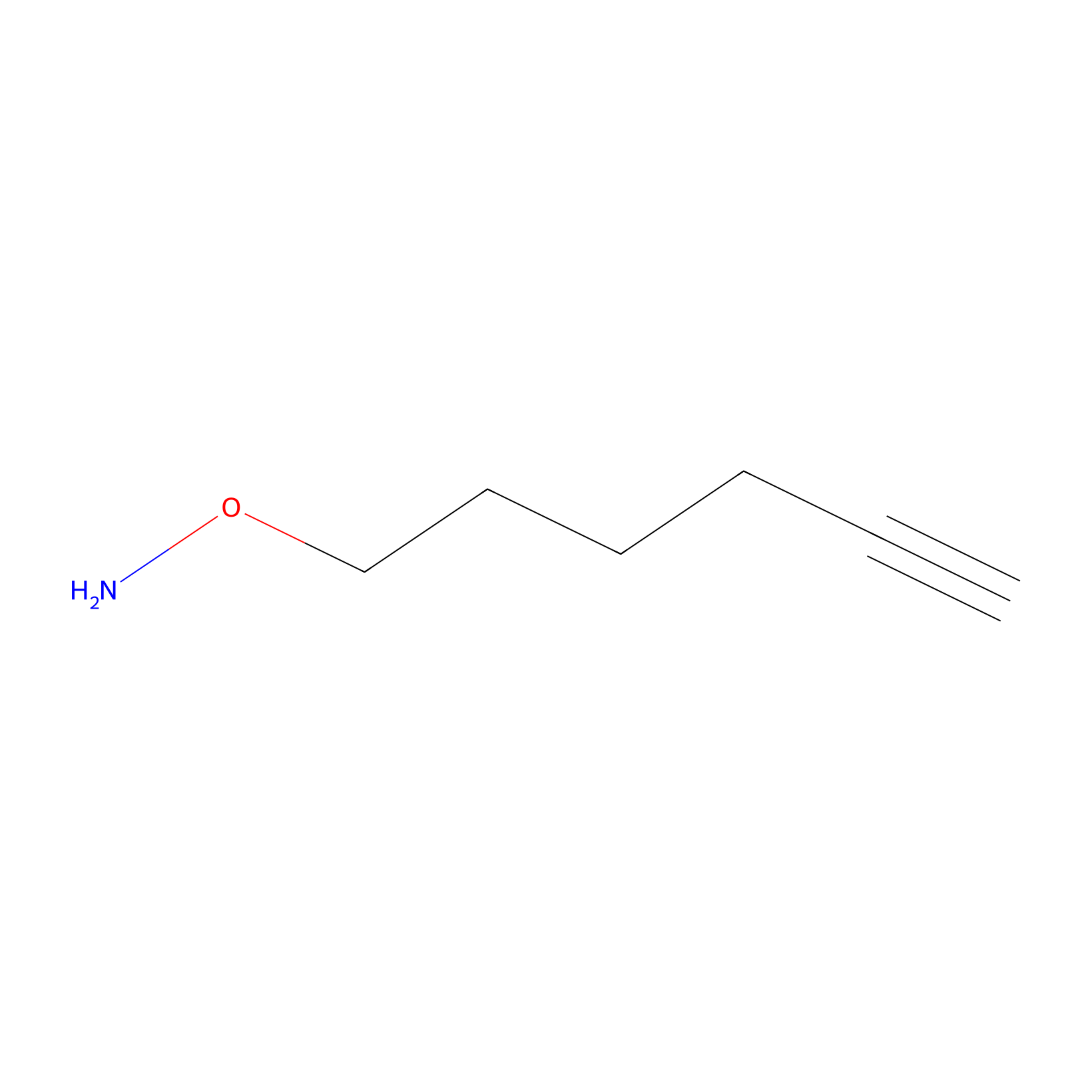 |
12.80 | LDD0443 | [25] | |
|
NAIA_5 Probe Info |
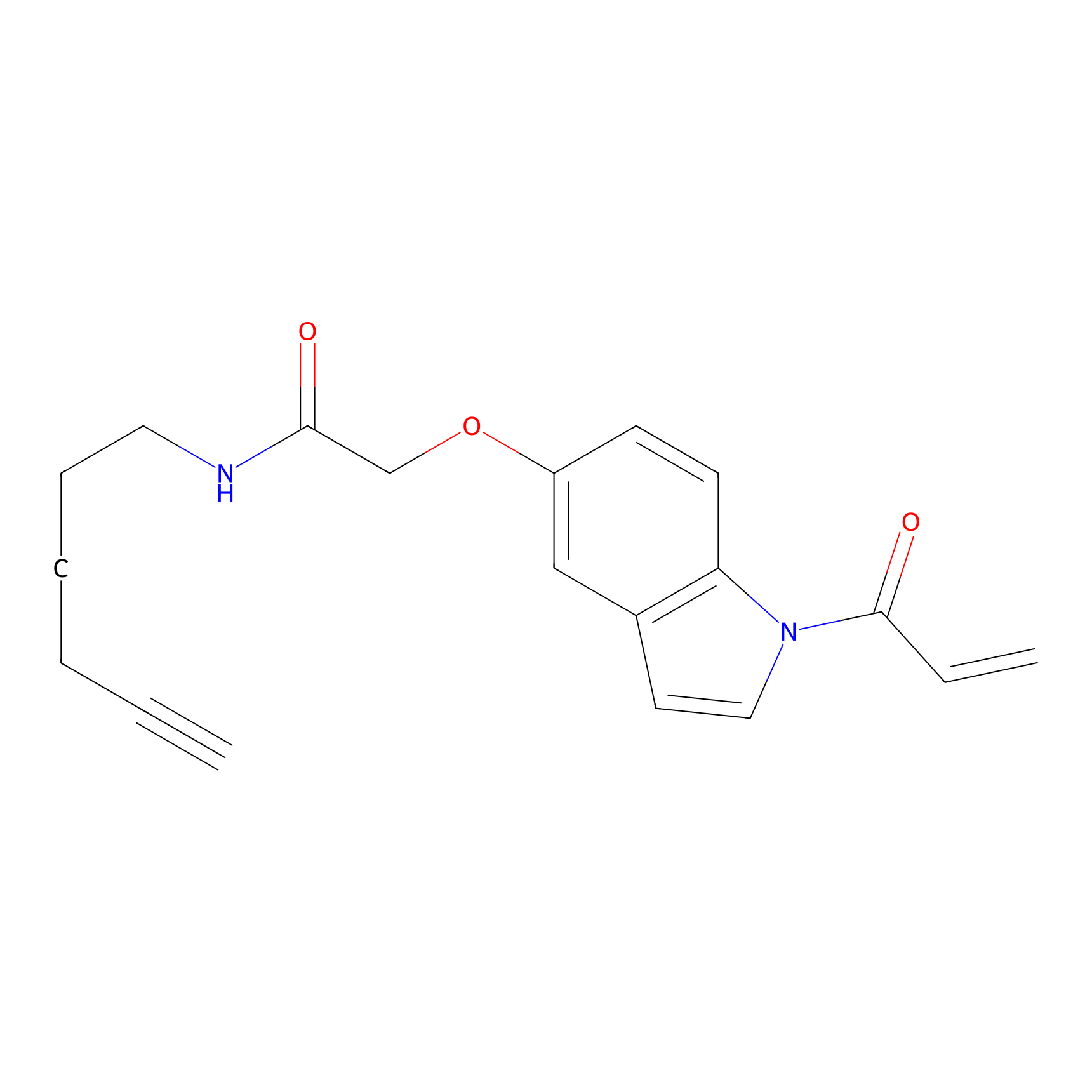 |
N.A. | LDD2223 | [15] | |
|
TER-AC Probe Info |
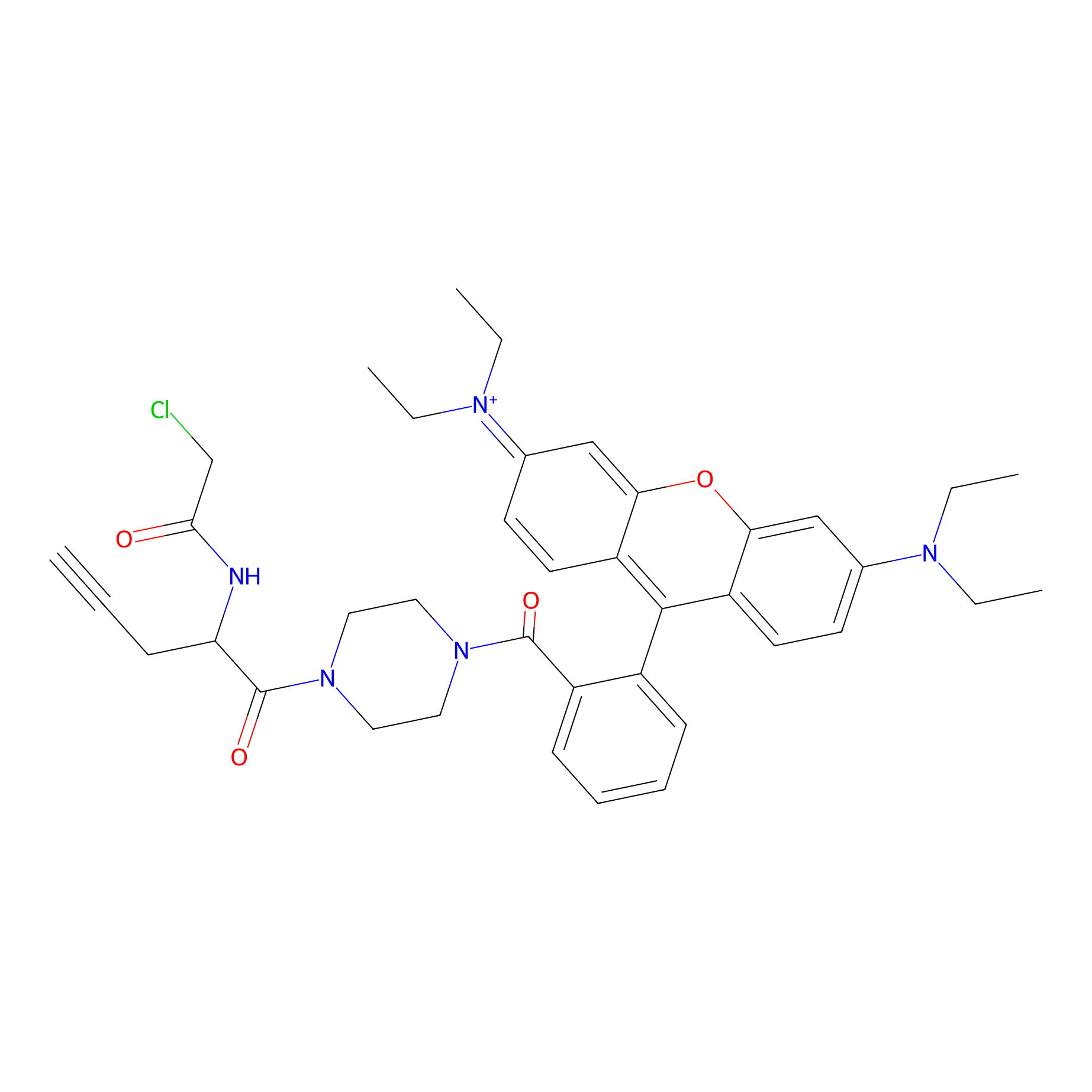 |
N.A. | LDD0426 | [26] | |
|
TPP-AC Probe Info |
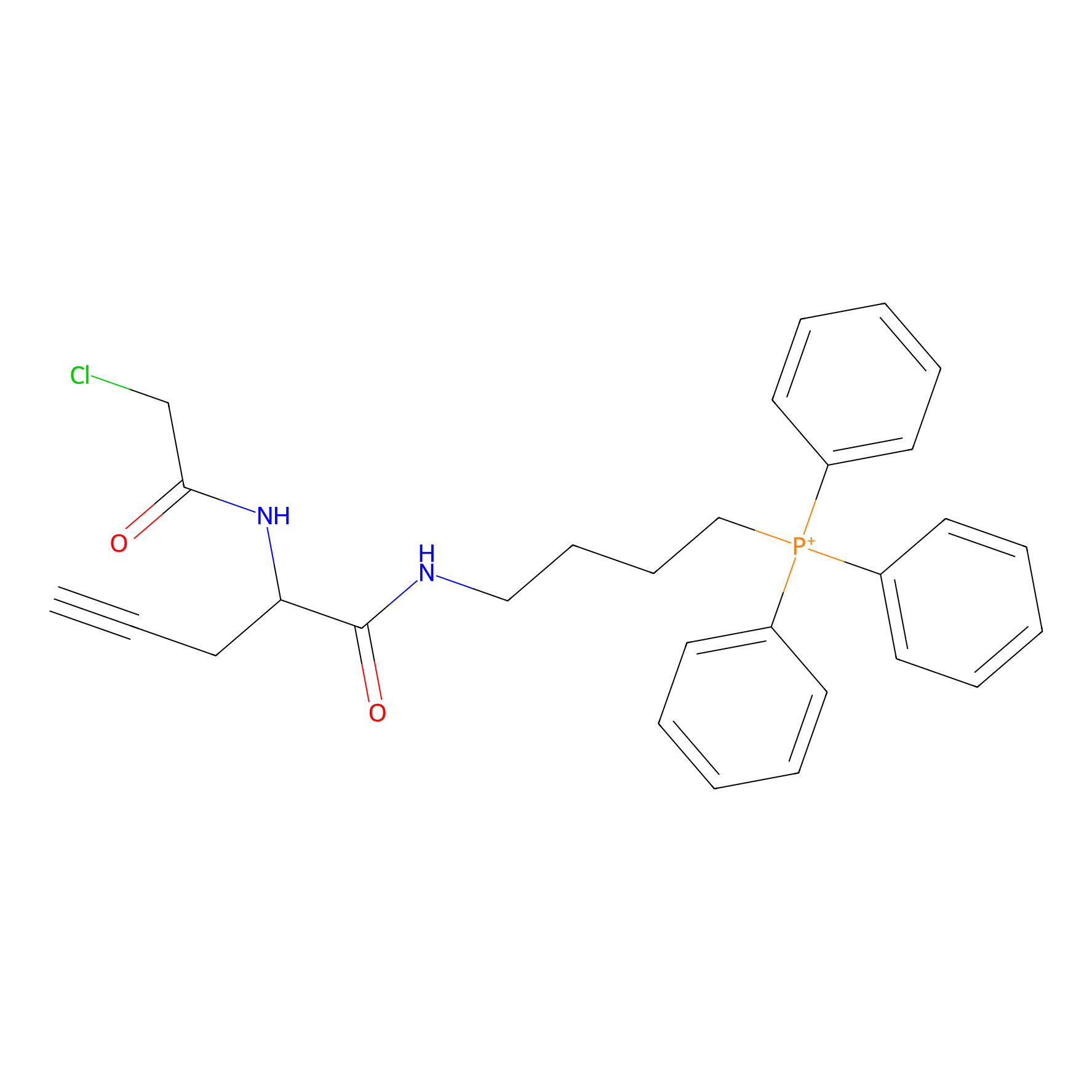 |
N.A. | LDD0427 | [26] | |
|
HHS-482 Probe Info |
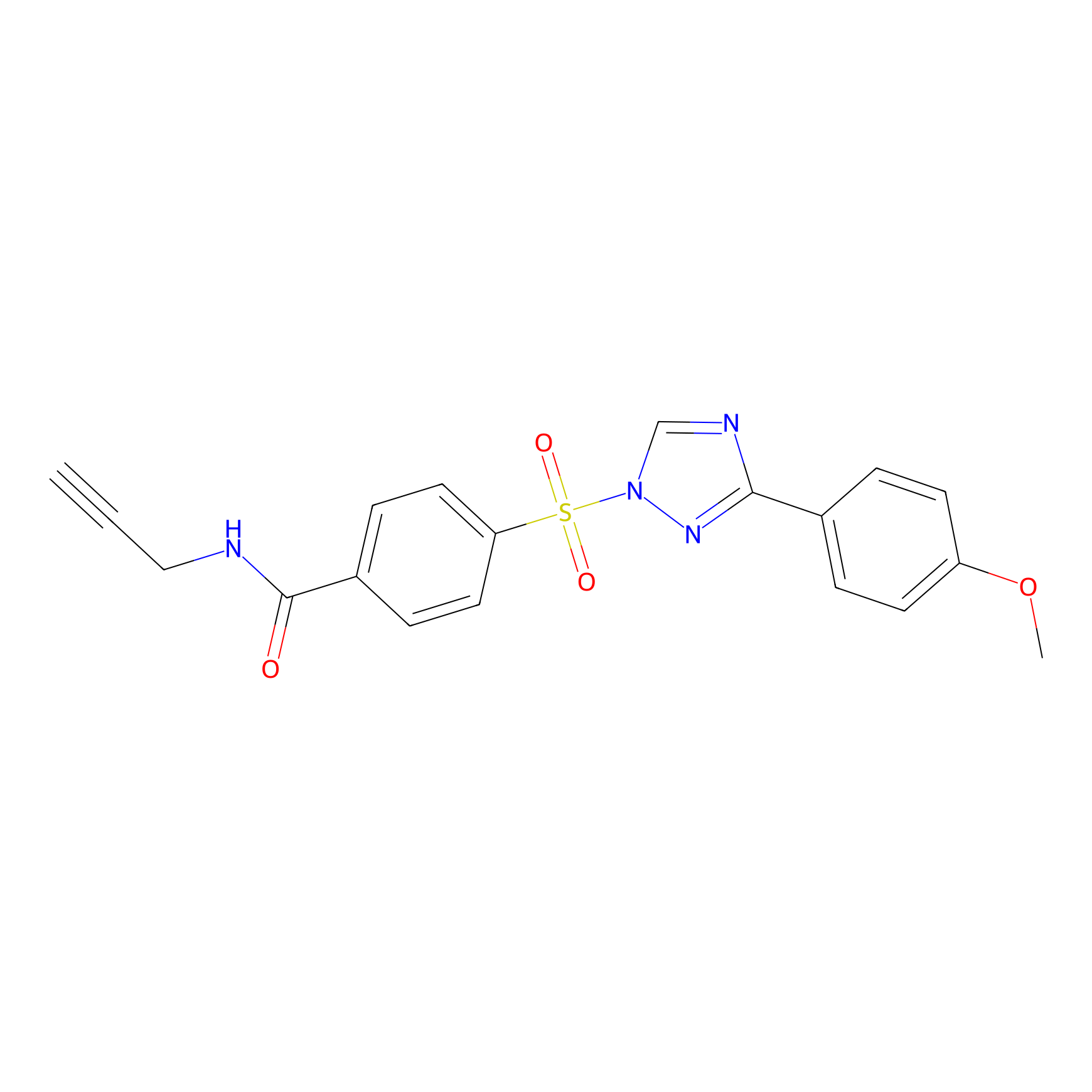 |
Y109(1.10) | LDD2239 | [27] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
STS-1 Probe Info |
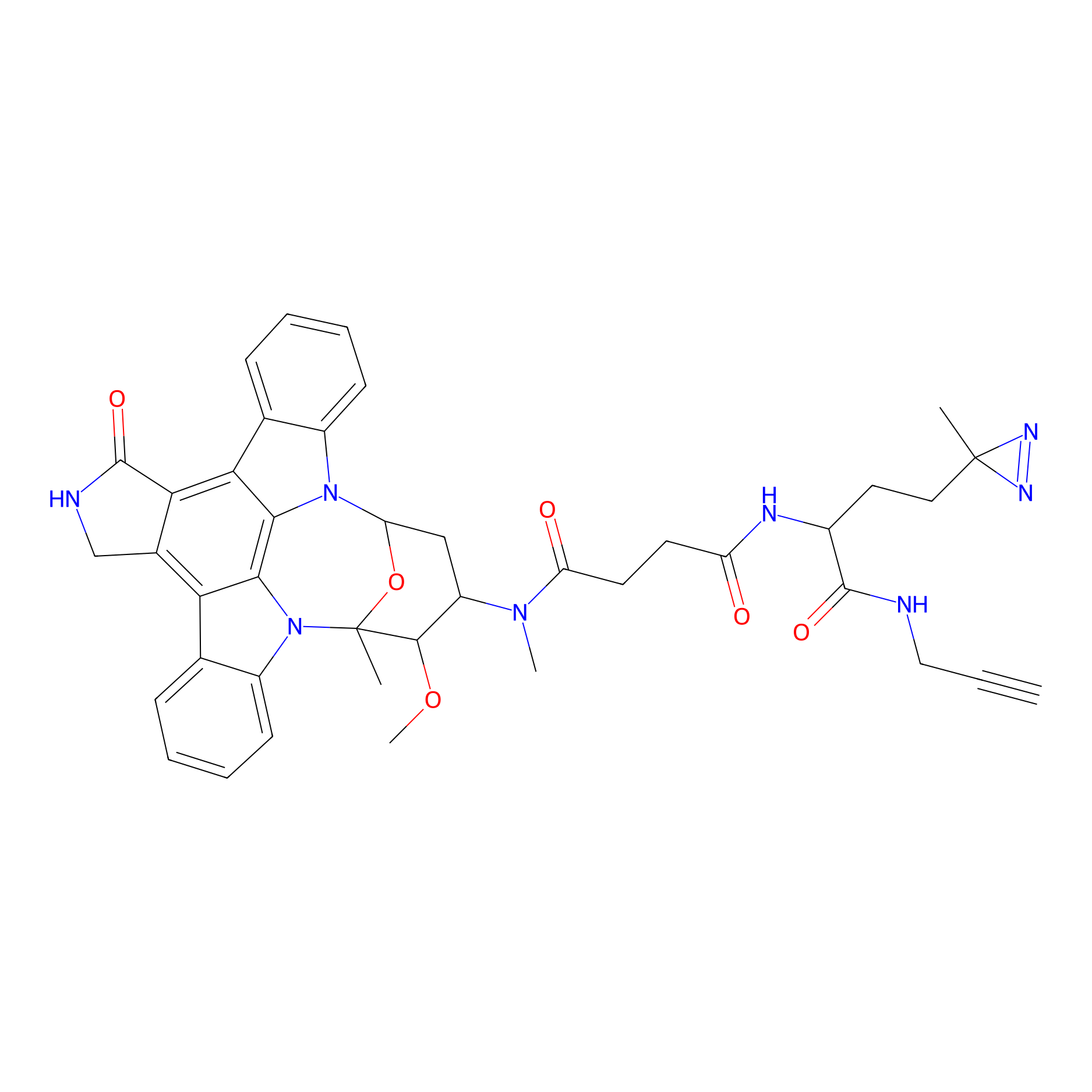 |
1.14 | LDD0137 | [28] | |
|
STS-2 Probe Info |
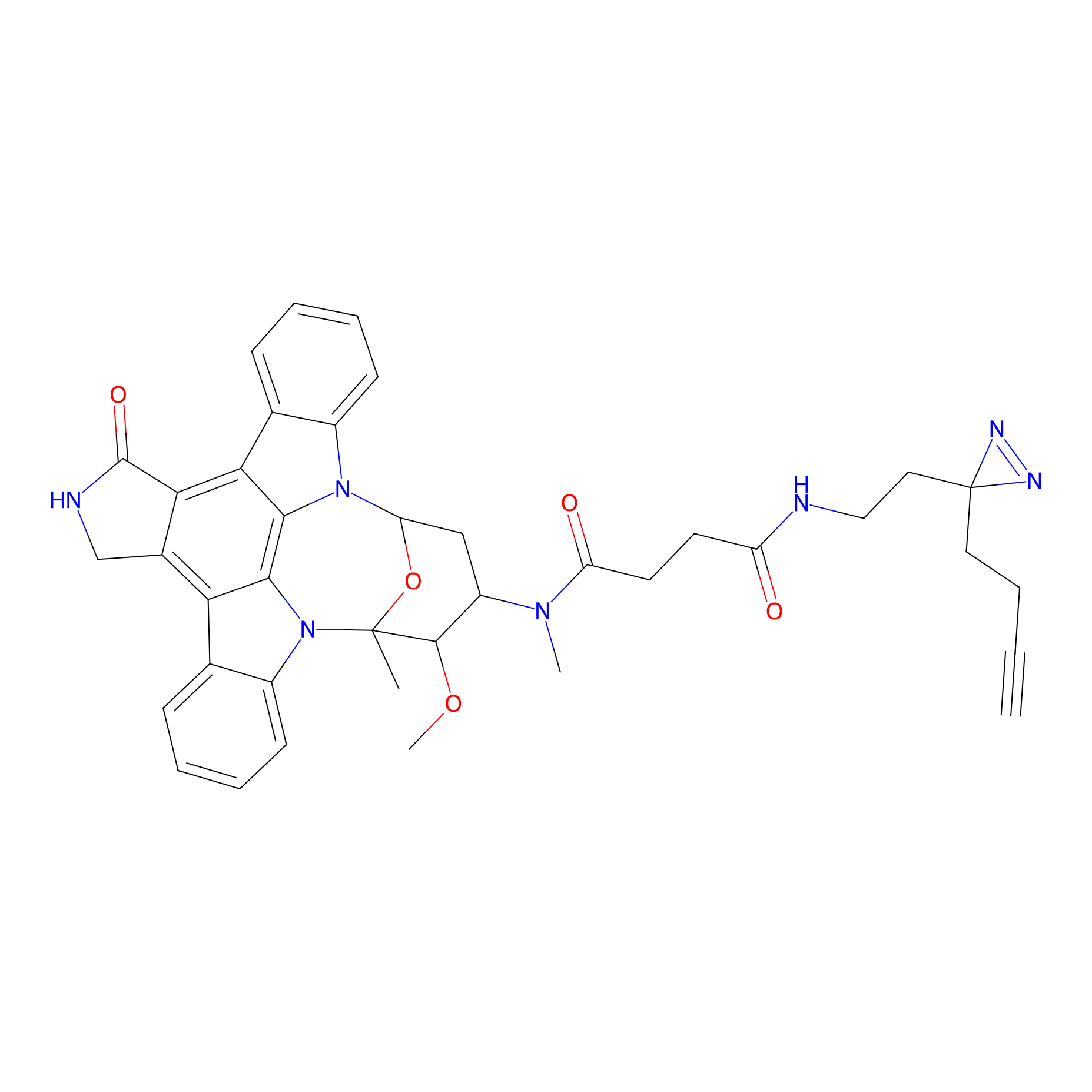 |
N.A. | LDD0138 | [28] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0502 | 1-(Cyanoacetyl)piperidine | MDA-MB-231 | C59(0.72) | LDD2095 | [6] |
| LDCM0537 | 2-Cyano-N,N-dimethylacetamide | MDA-MB-231 | C59(1.00) | LDD2130 | [6] |
| LDCM0524 | 2-Cyano-N-(2-morpholin-4-yl-ethyl)-acetamide | MDA-MB-231 | C59(0.92) | LDD2117 | [6] |
| LDCM0558 | 2-Cyano-N-phenylacetamide | MDA-MB-231 | C59(1.39) | LDD2152 | [6] |
| LDCM0510 | 3-(4-(Hydroxydiphenylmethyl)piperidin-1-yl)-3-oxopropanenitrile | MDA-MB-231 | C59(1.06) | LDD2103 | [6] |
| LDCM0539 | 3-(4-Isopropylpiperazin-1-yl)-3-oxopropanenitrile | MDA-MB-231 | C59(0.48) | LDD2132 | [6] |
| LDCM0538 | 4-(Cyanoacetyl)morpholine | MDA-MB-231 | C59(0.77) | LDD2131 | [6] |
| LDCM0545 | Acetamide | MDA-MB-231 | C59(0.46) | LDD2138 | [6] |
| LDCM0520 | AKOS000195272 | MDA-MB-231 | C59(0.82) | LDD2113 | [6] |
| LDCM0156 | Aniline | NCI-H1299 | 11.63 | LDD0403 | [1] |
| LDCM0498 | BS-3668 | MDA-MB-231 | C59(0.42) | LDD2091 | [6] |
| LDCM0108 | Chloroacetamide | HeLa | N.A. | LDD0222 | [23] |
| LDCM0632 | CL-Sc | Hep-G2 | C59(1.06); C59(0.90); C59(0.88); C59(0.51) | LDD2227 | [15] |
| LDCM0625 | F8 | Ramos | C59(1.33) | LDD2187 | [29] |
| LDCM0572 | Fragment10 | Ramos | C59(0.79) | LDD2189 | [29] |
| LDCM0573 | Fragment11 | Ramos | C59(0.00) | LDD2190 | [29] |
| LDCM0574 | Fragment12 | Ramos | C59(0.81) | LDD2191 | [29] |
| LDCM0575 | Fragment13 | Ramos | C59(0.85) | LDD2192 | [29] |
| LDCM0576 | Fragment14 | Ramos | C59(0.76) | LDD2193 | [29] |
| LDCM0579 | Fragment20 | Ramos | C59(0.57) | LDD2194 | [29] |
| LDCM0580 | Fragment21 | Ramos | C59(1.00) | LDD2195 | [29] |
| LDCM0582 | Fragment23 | Ramos | C59(1.84) | LDD2196 | [29] |
| LDCM0578 | Fragment27 | Ramos | C59(1.15) | LDD2197 | [29] |
| LDCM0586 | Fragment28 | Ramos | C59(0.92) | LDD2198 | [29] |
| LDCM0588 | Fragment30 | Ramos | C59(0.82) | LDD2199 | [29] |
| LDCM0589 | Fragment31 | Ramos | C59(0.90) | LDD2200 | [29] |
| LDCM0590 | Fragment32 | Ramos | C59(0.64) | LDD2201 | [29] |
| LDCM0468 | Fragment33 | Ramos | C59(0.72) | LDD2202 | [29] |
| LDCM0596 | Fragment38 | Ramos | C59(0.82) | LDD2203 | [29] |
| LDCM0566 | Fragment4 | Ramos | C59(0.71) | LDD2184 | [29] |
| LDCM0610 | Fragment52 | Ramos | C59(0.68) | LDD2204 | [29] |
| LDCM0614 | Fragment56 | Ramos | C59(0.79) | LDD2205 | [29] |
| LDCM0569 | Fragment7 | Ramos | C59(0.62) | LDD2186 | [29] |
| LDCM0571 | Fragment9 | Ramos | C59(0.56) | LDD2188 | [29] |
| LDCM0022 | KB02 | Ramos | C59(0.53) | LDD2182 | [29] |
| LDCM0023 | KB03 | Ramos | C59(0.69) | LDD2183 | [29] |
| LDCM0024 | KB05 | Ramos | C59(1.36) | LDD2185 | [29] |
| LDCM0509 | N-(4-bromo-3,5-dimethylphenyl)-2-nitroacetamide | MDA-MB-231 | C59(1.39) | LDD2102 | [6] |
| LDCM0496 | Nucleophilic fragment 11a | MDA-MB-231 | C59(0.61) | LDD2089 | [6] |
| LDCM0497 | Nucleophilic fragment 11b | MDA-MB-231 | C59(1.23) | LDD2090 | [6] |
| LDCM0499 | Nucleophilic fragment 12b | MDA-MB-231 | C59(1.60) | LDD2092 | [6] |
| LDCM0500 | Nucleophilic fragment 13a | MDA-MB-231 | C59(1.25) | LDD2093 | [6] |
| LDCM0501 | Nucleophilic fragment 13b | MDA-MB-231 | C59(2.43) | LDD2094 | [6] |
| LDCM0503 | Nucleophilic fragment 14b | MDA-MB-231 | C59(0.43) | LDD2096 | [6] |
| LDCM0504 | Nucleophilic fragment 15a | MDA-MB-231 | C59(0.84) | LDD2097 | [6] |
| LDCM0505 | Nucleophilic fragment 15b | MDA-MB-231 | C59(0.22) | LDD2098 | [6] |
| LDCM0506 | Nucleophilic fragment 16a | MDA-MB-231 | C59(0.88) | LDD2099 | [6] |
| LDCM0507 | Nucleophilic fragment 16b | MDA-MB-231 | C59(0.53) | LDD2100 | [6] |
| LDCM0508 | Nucleophilic fragment 17a | MDA-MB-231 | C59(0.83) | LDD2101 | [6] |
| LDCM0512 | Nucleophilic fragment 19a | MDA-MB-231 | C59(1.43) | LDD2105 | [6] |
| LDCM0514 | Nucleophilic fragment 20a | MDA-MB-231 | C59(1.01) | LDD2107 | [6] |
| LDCM0515 | Nucleophilic fragment 20b | MDA-MB-231 | C59(0.14) | LDD2108 | [6] |
| LDCM0516 | Nucleophilic fragment 21a | MDA-MB-231 | C59(0.57) | LDD2109 | [6] |
| LDCM0518 | Nucleophilic fragment 22a | MDA-MB-231 | C59(1.01) | LDD2111 | [6] |
| LDCM0523 | Nucleophilic fragment 24b | MDA-MB-231 | C59(0.91) | LDD2116 | [6] |
| LDCM0525 | Nucleophilic fragment 25b | MDA-MB-231 | C59(0.72) | LDD2118 | [6] |
| LDCM0526 | Nucleophilic fragment 26a | MDA-MB-231 | C59(2.73) | LDD2119 | [6] |
| LDCM0527 | Nucleophilic fragment 26b | MDA-MB-231 | C59(0.95) | LDD2120 | [6] |
| LDCM0529 | Nucleophilic fragment 27b | MDA-MB-231 | C59(0.79) | LDD2122 | [6] |
| LDCM0530 | Nucleophilic fragment 28a | MDA-MB-231 | C59(0.57) | LDD2123 | [6] |
| LDCM0532 | Nucleophilic fragment 29a | MDA-MB-231 | C59(0.74) | LDD2125 | [6] |
| LDCM0533 | Nucleophilic fragment 29b | MDA-MB-231 | C59(0.62) | LDD2126 | [6] |
| LDCM0534 | Nucleophilic fragment 30a | MDA-MB-231 | C59(0.87) | LDD2127 | [6] |
| LDCM0535 | Nucleophilic fragment 30b | MDA-MB-231 | C59(1.00) | LDD2128 | [6] |
| LDCM0536 | Nucleophilic fragment 31 | MDA-MB-231 | C59(1.15) | LDD2129 | [6] |
| LDCM0540 | Nucleophilic fragment 35 | MDA-MB-231 | C59(0.62) | LDD2133 | [6] |
| LDCM0541 | Nucleophilic fragment 36 | MDA-MB-231 | C59(0.46) | LDD2134 | [6] |
| LDCM0542 | Nucleophilic fragment 37 | MDA-MB-231 | C59(0.98) | LDD2135 | [6] |
| LDCM0543 | Nucleophilic fragment 38 | MDA-MB-231 | C59(1.05) | LDD2136 | [6] |
| LDCM0544 | Nucleophilic fragment 39 | MDA-MB-231 | C59(0.83) | LDD2137 | [6] |
| LDCM0211 | Nucleophilic fragment 3b | MDA-MB-231 | C59(2.05) | LDD1700 | [6] |
| LDCM0546 | Nucleophilic fragment 40 | MDA-MB-231 | C59(0.72) | LDD2140 | [6] |
| LDCM0549 | Nucleophilic fragment 43 | MDA-MB-231 | C59(0.97) | LDD2143 | [6] |
| LDCM0550 | Nucleophilic fragment 5a | MDA-MB-231 | C59(3.28) | LDD2144 | [6] |
| LDCM0552 | Nucleophilic fragment 6a | MDA-MB-231 | C59(0.76) | LDD2146 | [6] |
| LDCM0553 | Nucleophilic fragment 6b | MDA-MB-231 | C59(3.20) | LDD2147 | [6] |
| LDCM0554 | Nucleophilic fragment 7a | MDA-MB-231 | C59(0.79) | LDD2148 | [6] |
| LDCM0555 | Nucleophilic fragment 7b | MDA-MB-231 | C59(0.68) | LDD2149 | [6] |
| LDCM0556 | Nucleophilic fragment 8a | MDA-MB-231 | C59(0.39) | LDD2150 | [6] |
| LDCM0557 | Nucleophilic fragment 8b | MDA-MB-231 | C59(0.78) | LDD2151 | [6] |
| LDCM0559 | Nucleophilic fragment 9b | MDA-MB-231 | C59(1.10) | LDD2153 | [6] |
| LDCM0131 | RA190 | MM1.R | C59(1.39) | LDD0304 | [30] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Transcription factor
Other
References
