Details of the Target
General Information of Target
Target Site Mutations in Different Cell Lines
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
DBIA Probe Info |
 |
C275(1.72) | LDD3397 | [1] | |
|
Johansson_61 Probe Info |
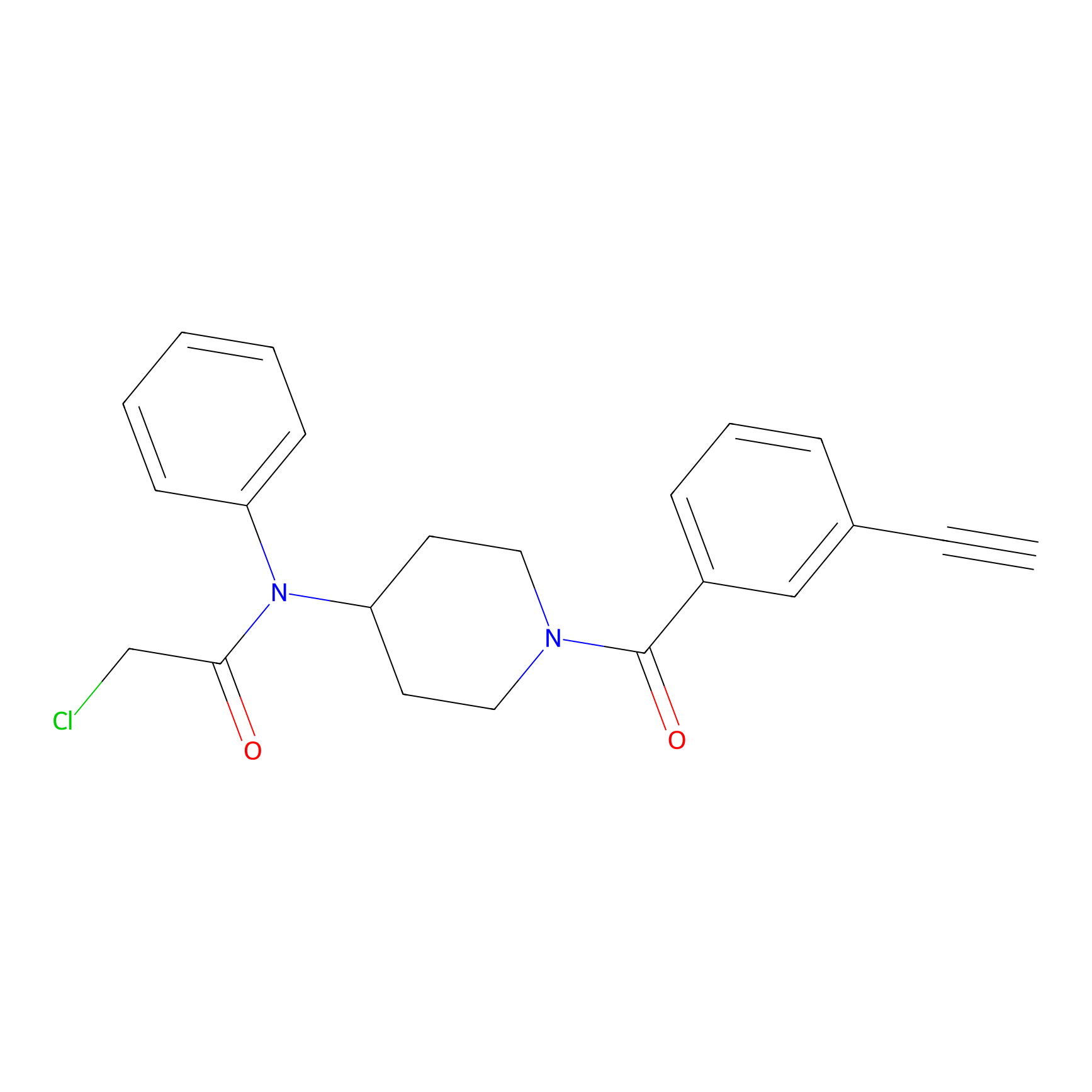 |
_(20.00) | LDD1485 | [2] | |
|
Alkyne-RA190 Probe Info |
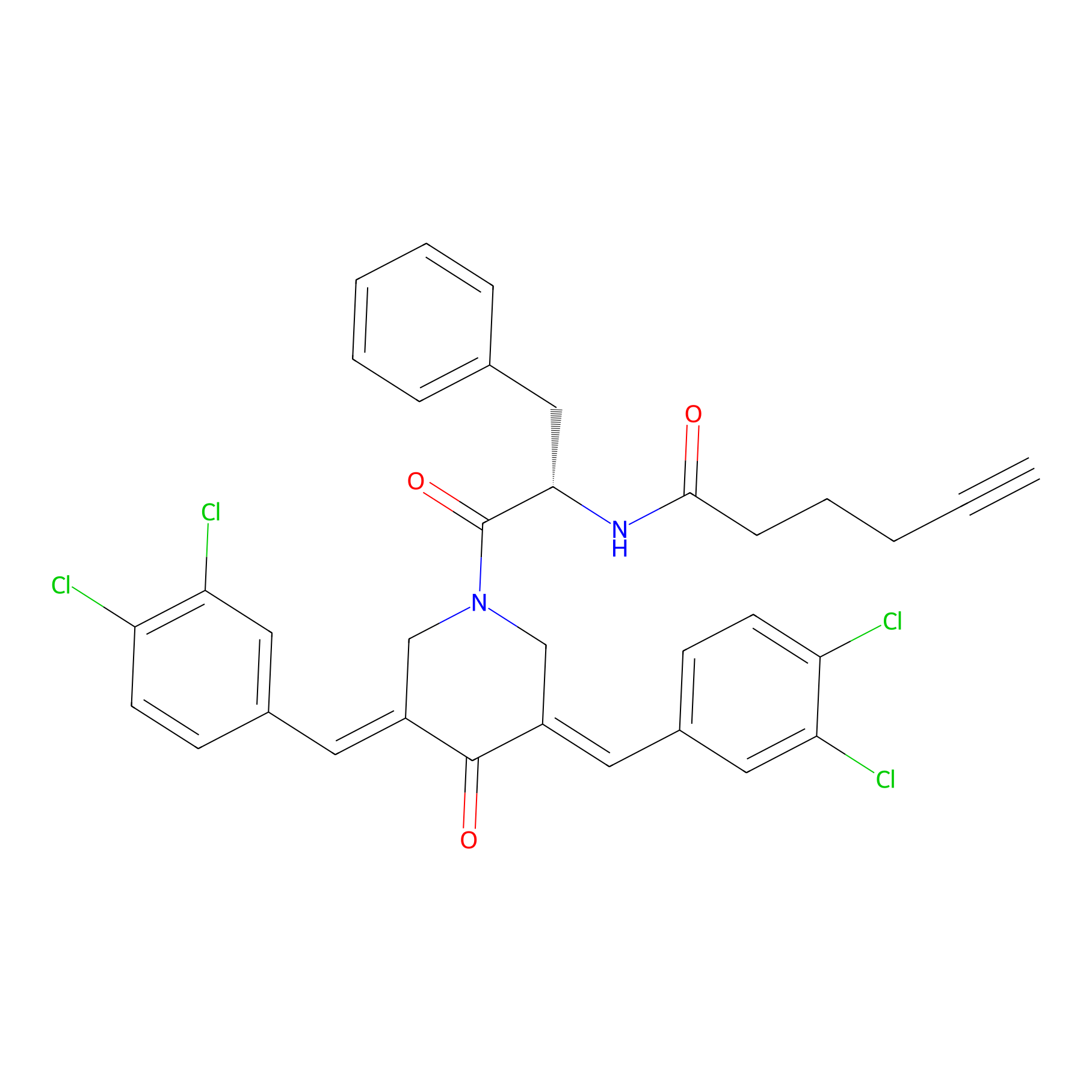 |
4.08 | LDD0299 | [3] | |
|
4-Iodoacetamidophenylacetylene Probe Info |
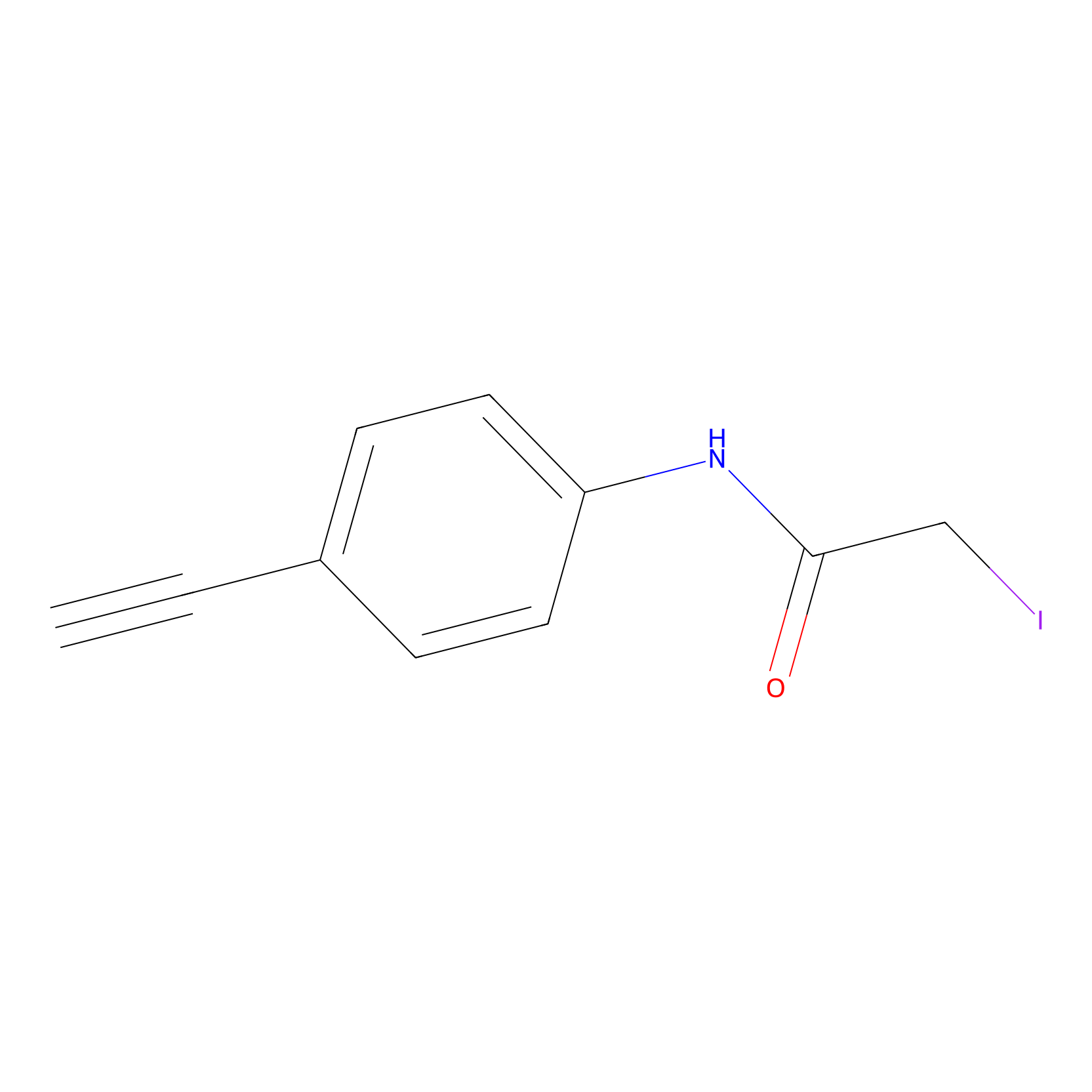 |
C180(0.00); C254(0.00); C82(0.00); C173(0.00) | LDD0038 | [4] | |
|
IA-alkyne Probe Info |
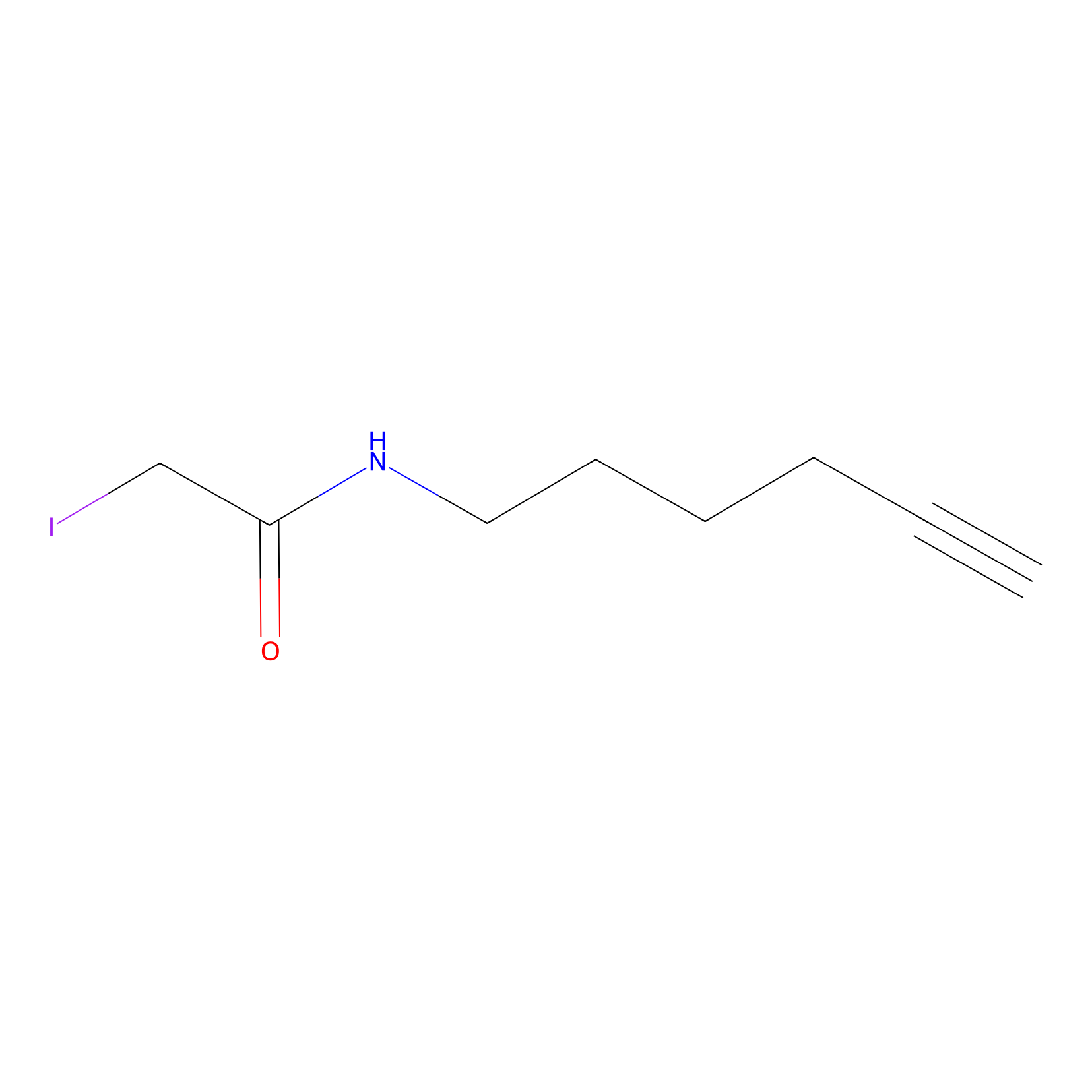 |
C180(0.00); C254(0.00); C82(0.00); C173(0.00) | LDD0036 | [4] | |
|
Lodoacetamide azide Probe Info |
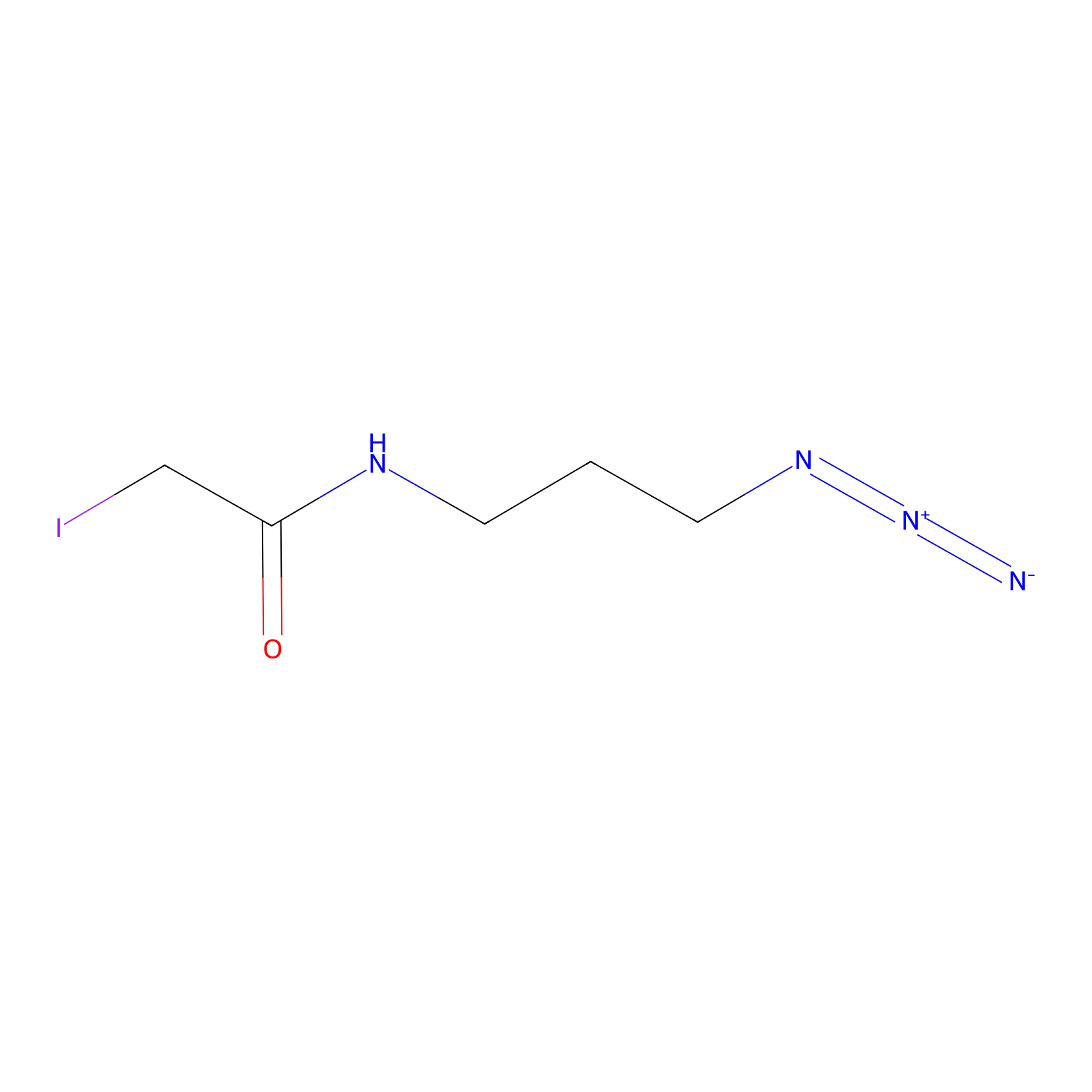 |
C180(0.00); C254(0.00); C82(0.00); C173(0.00) | LDD0037 | [4] | |
|
Compound 10 Probe Info |
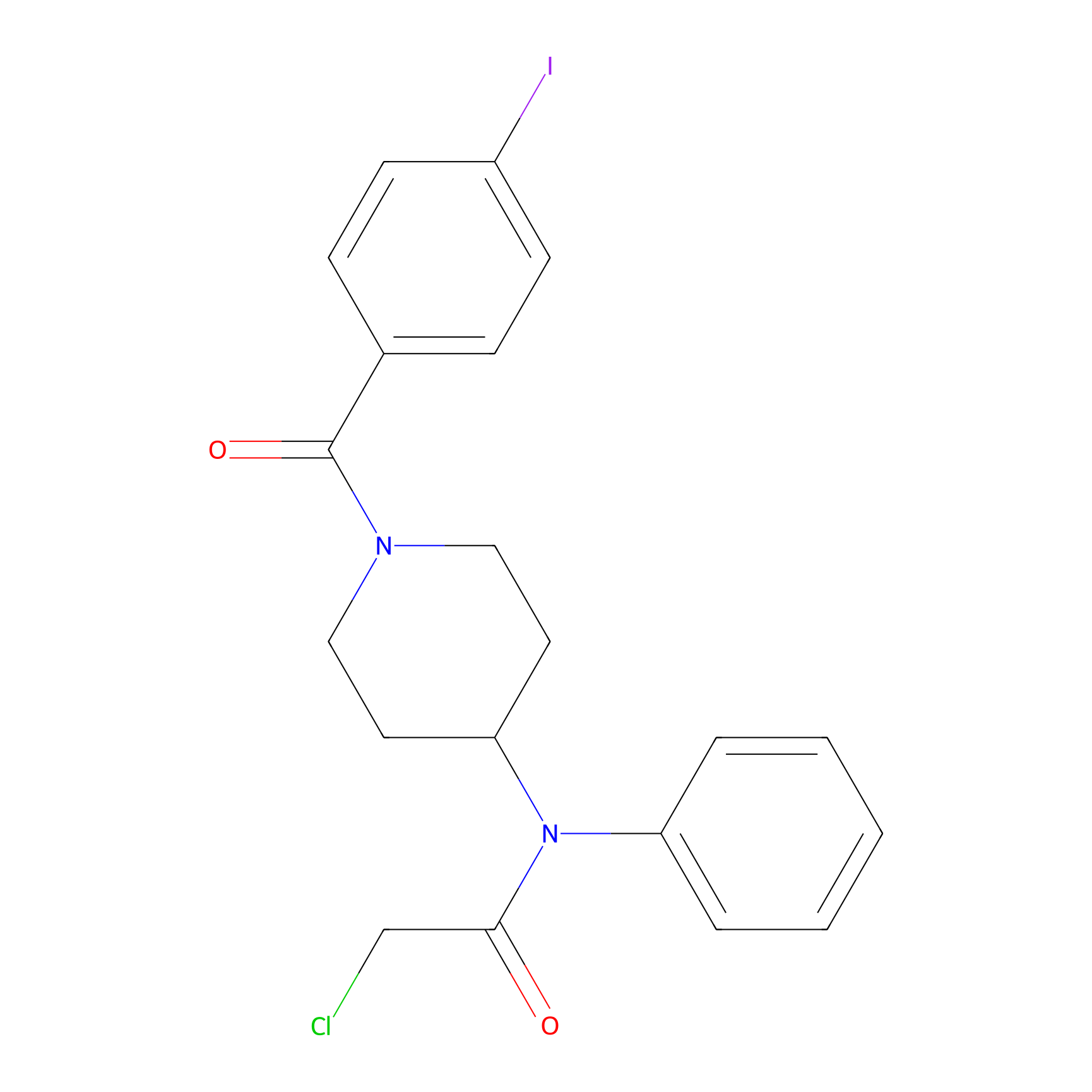 |
N.A. | LDD2216 | [5] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0201 | EV-3 | T cell | C287(4.61) | LDD0515 | [6] |
| LDCM0202 | EV-93 | T cell | C287(6.49) | LDD0514 | [6] |
| LDCM0203 | EV-96 | T cell | C275(4.06) | LDD0523 | [6] |
| LDCM0206 | EV-99 | T cell | C296(6.50) | LDD0521 | [6] |
| LDCM0625 | F8 | Ramos | 1.43 | LDD2187 | [7] |
| LDCM0578 | Fragment27 | Ramos | 1.07 | LDD2197 | [7] |
| LDCM0586 | Fragment28 | Ramos | 1.85 | LDD2198 | [7] |
| LDCM0616 | Fragment61 | Jurkat | _(20.00) | LDD1489 | [2] |
| LDCM0615 | Fragment63-R | Jurkat | _(8.16) | LDD1487 | [2] |
| LDCM0569 | Fragment7 | Jurkat | _(20.00) | LDD1485 | [2] |
| LDCM0022 | KB02 | Ramos | 20.00 | LDD0431 | [8] |
| LDCM0023 | KB03 | 697 | C180(2.90); C119(3.07) | LDD2662 | [1] |
| LDCM0024 | KB05 | PF-382 | C275(1.72) | LDD3397 | [1] |
| LDCM0169 | KB63 | Ramos | 7.90 | LDD0432 | [8] |
| LDCM0131 | RA190 | MM1.R | 4.08 | LDD0299 | [3] |
| LDCM0170 | Structure8 | Ramos | 20.00; 5.86 | LDD0433 | [8] |
The Interaction Atlas With This Target
The Drug(s) Related To This Target
Approved
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Daratumumab | Antibody | D0RV4P | |||
| Isatuximab | . | DB14811 | |||
Phase 2
Phase 1
Clinical trial
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Car-t Cells Targeting Cd38 | CAR T Cell Therapy | D0V6WU | |||
Investigative
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Humax-cd38b | . | D0N1QH | |||
| Mezagitamab | . | DB16370 | |||
References
