Details of the Target
General Information of Target
| Target ID | LDTP05817 | |||||
|---|---|---|---|---|---|---|
| Target Name | Voltage-gated potassium channel subunit beta-2 (KCNAB2) | |||||
| Gene Name | KCNAB2 | |||||
| Gene ID | 8514 | |||||
| Synonyms |
KCNA2B; KCNK2; Voltage-gated potassium channel subunit beta-2; EC 1.1.1.-; K(+) channel subunit beta-2; Kv-beta-2; hKvbeta2 |
|||||
| 3D Structure | ||||||
| Sequence |
MYPESTTGSPARLSLRQTGSPGMIYSTRYGSPKRQLQFYRNLGKSGLRVSCLGLGTWVTF
GGQITDEMAEQLMTLAYDNGINLFDTAEVYAAGKAEVVLGNIIKKKGWRRSSLVITTKIF WGGKAETERGLSRKHIIEGLKASLERLQLEYVDVVFANRPDPNTPMEETVRAMTHVINQG MAMYWGTSRWSSMEIMEAYSVARQFNLTPPICEQAEYHMFQREKVEVQLPELFHKIGVGA MTWSPLACGIVSGKYDSGIPPYSRASLKGYQWLKDKILSEEGRRQQAKLKELQAIAERLG CTLPQLAIAWCLRNEGVSSVLLGASNADQLMENIGAIQVLPKLSSSIIHEIDSILGNKPY SKKDYRS |
|||||
| Target Bioclass |
Transporter and channel
|
|||||
| Family |
Shaker potassium channel beta subunit family
|
|||||
| Subcellular location |
Cytoplasm
|
|||||
| Function |
Cytoplasmic potassium channel subunit that modulates the characteristics of the channel-forming alpha-subunits. Contributes to the regulation of nerve signaling, and prevents neuronal hyperexcitability. Promotes expression of the pore-forming alpha subunits at the cell membrane, and thereby increases channel activity. Promotes potassium channel closure via a mechanism that does not involve physical obstruction of the channel pore. Promotes KCNA4 channel closure. Modulates the functional properties of KCNA5. Enhances KCNB2 channel activity. Binds NADPH and has NADPH-dependent aldoketoreductase activity. Has broad substrate specificity and can catalyze the reduction of methylglyoxal, 9,10-phenanthrenequinone, prostaglandin J2, 4-nitrobenzaldehyde, 4-nitroacetophenone and 4-oxo-trans-2-nonenal (in vitro).
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
TH211 Probe Info |
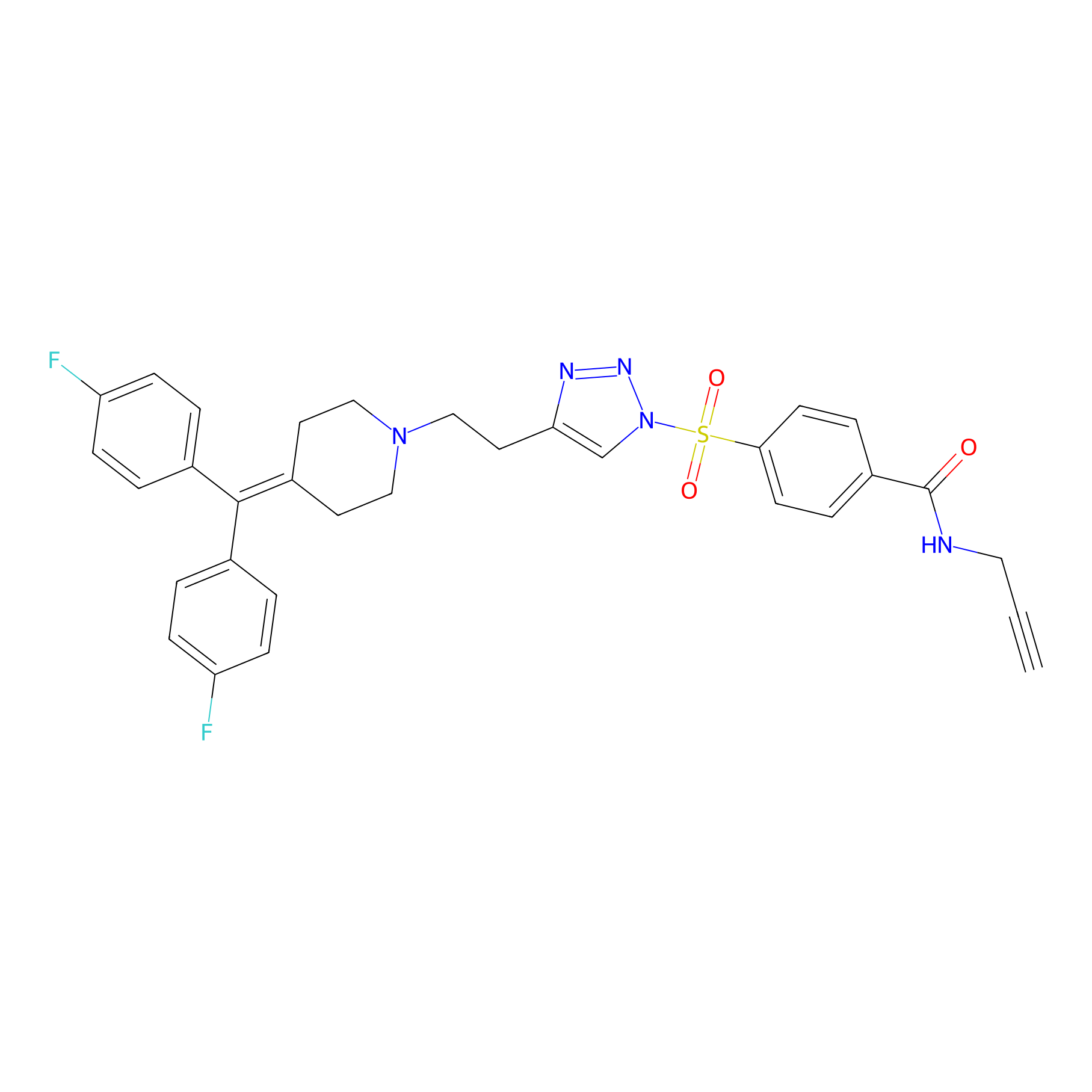 |
Y360(20.00); Y270(8.42) | LDD0260 | [1] | |
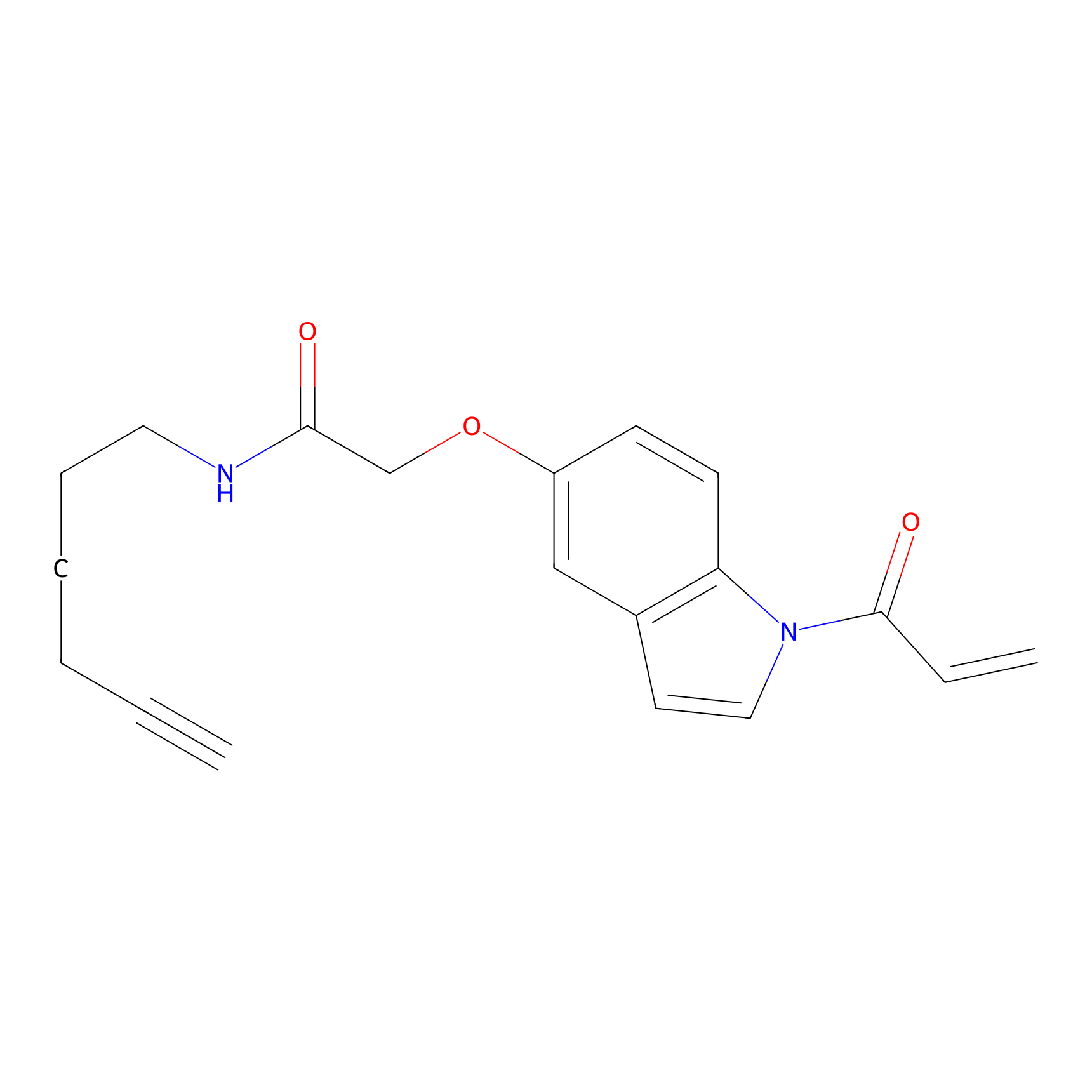
|
STPyne Probe Info |
 |
K124(20.00) | LDD2219 | [2] | |
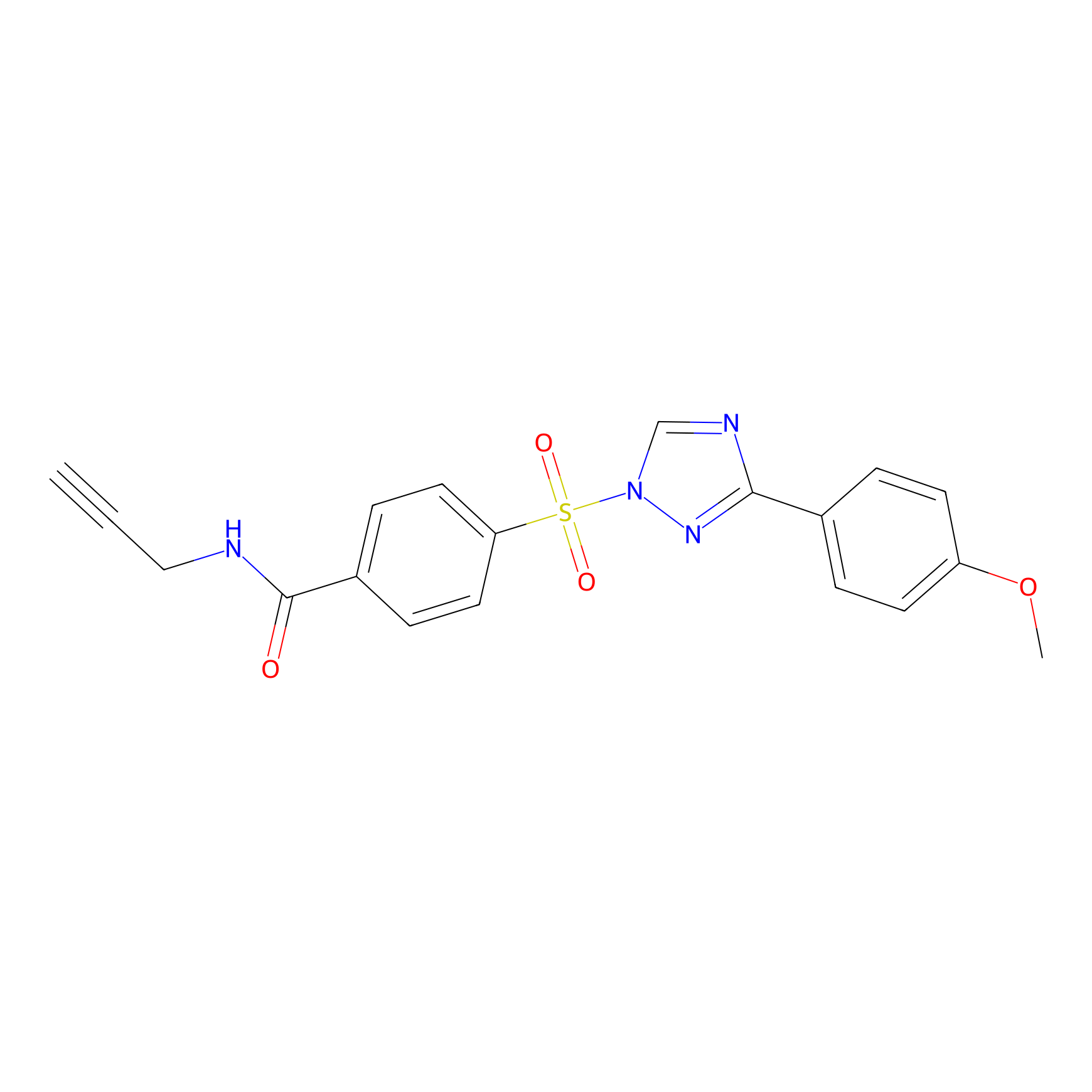
|
NAIA_5 Probe Info |
 |
C248(20.00) | LDD2227 | [3] | |
|
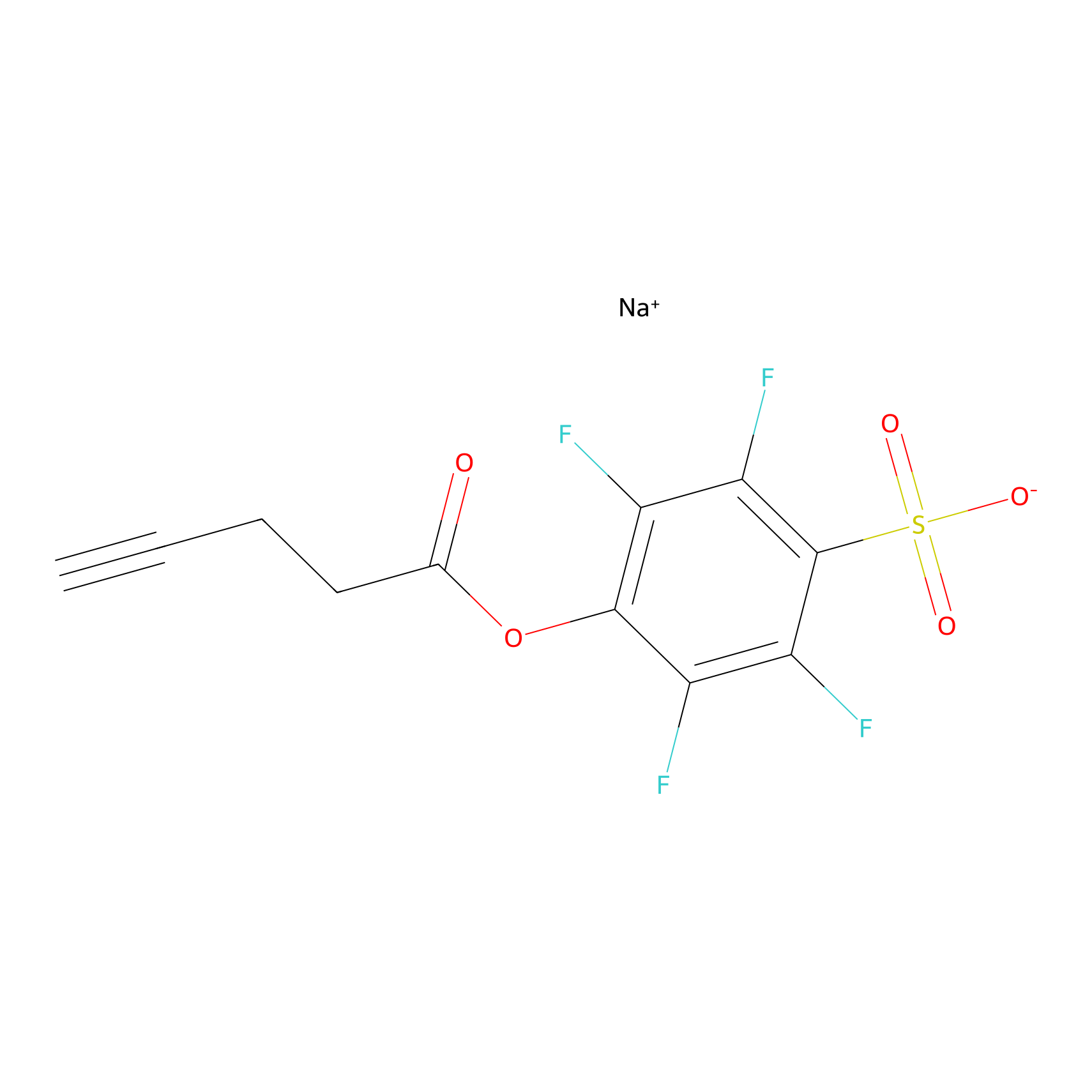
DBIA Probe Info |
 |
C248(27.72) | LDD0209 | [4] | |
|
HHS-482 Probe Info |
 |
Y360(1.02) | LDD0285 | [5] | |
|
HHS-475 Probe Info |
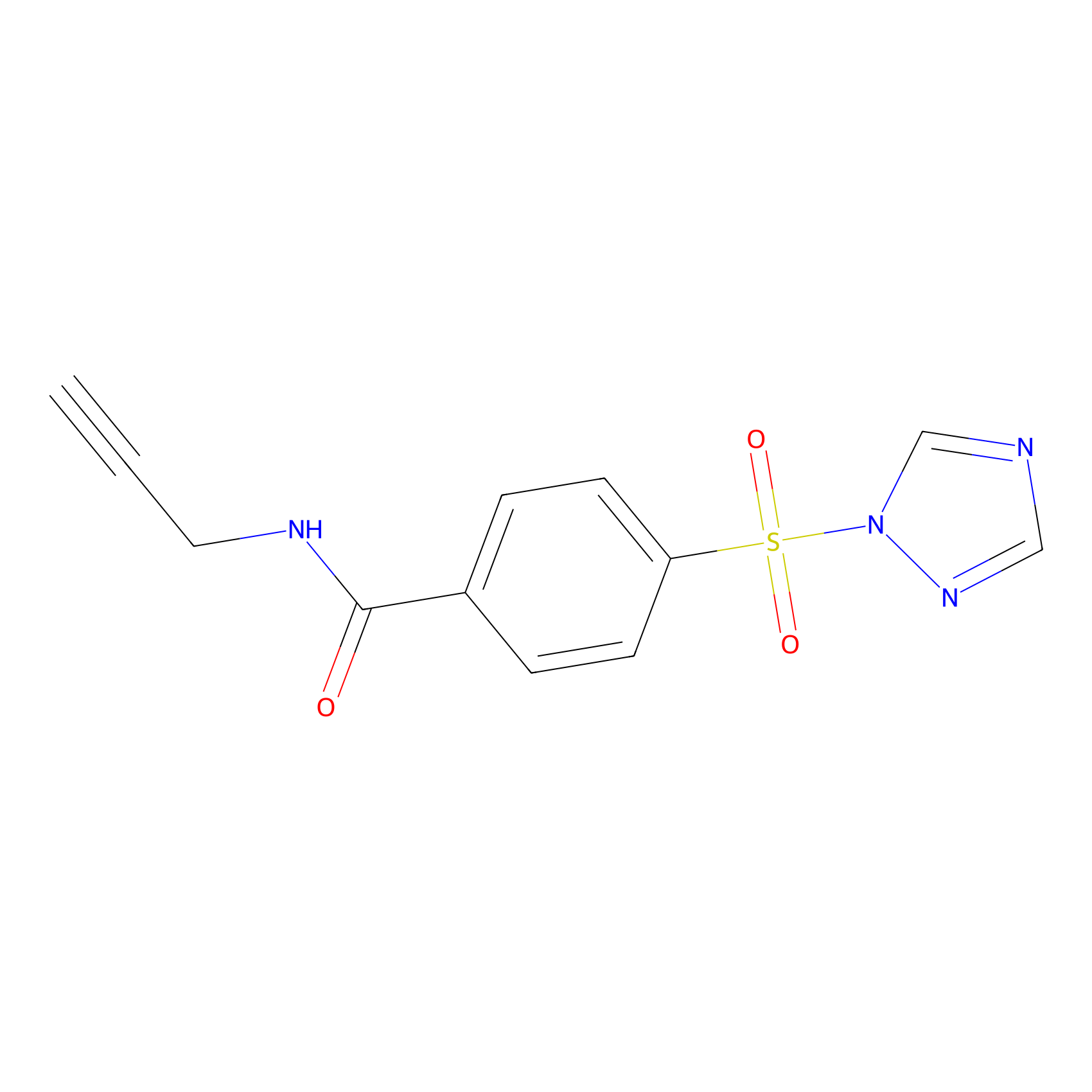 |
Y360(1.51) | LDD0264 | [6] | |
|
4-Iodoacetamidophenylacetylene Probe Info |
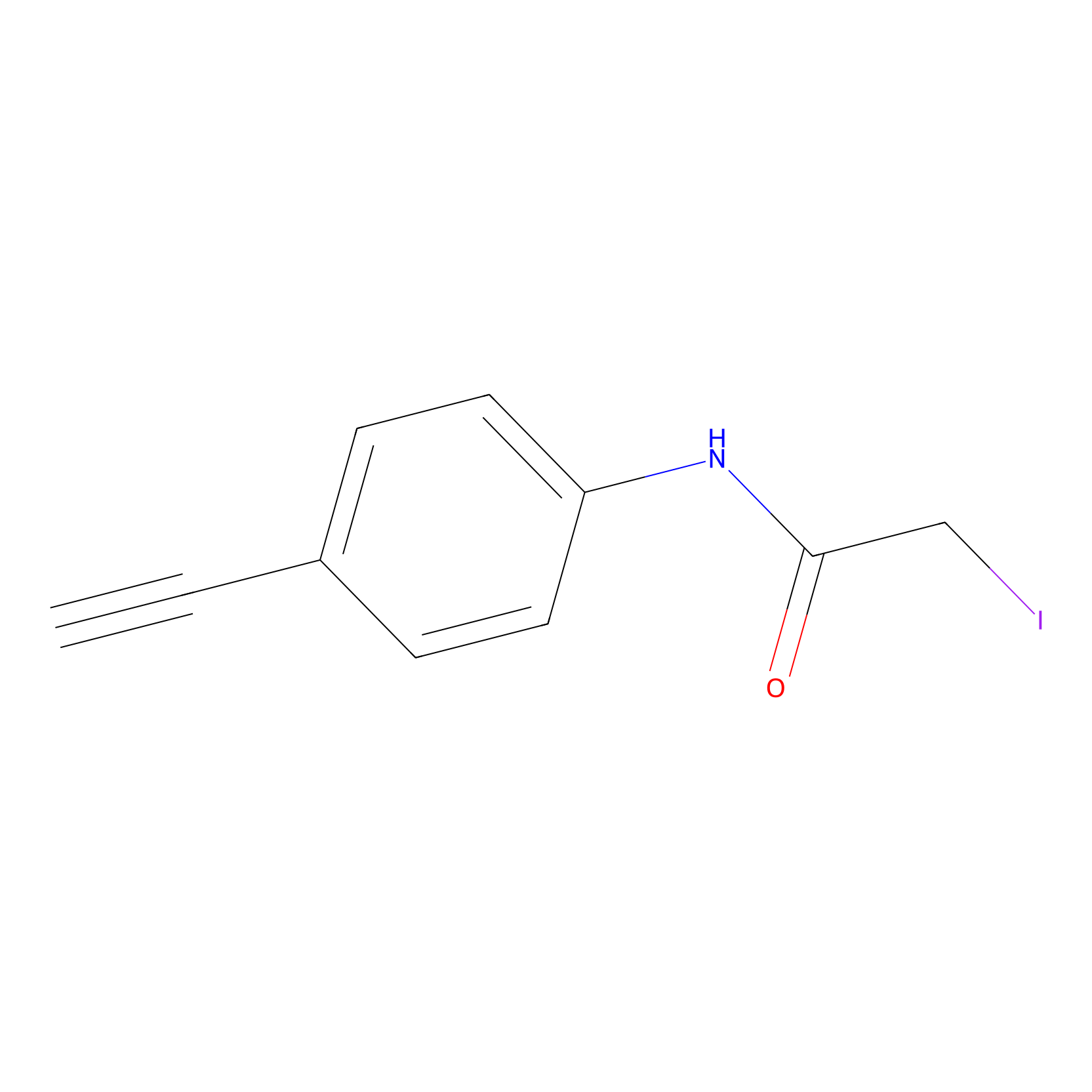 |
N.A. | LDD0038 | [7] | |
|
IA-alkyne Probe Info |
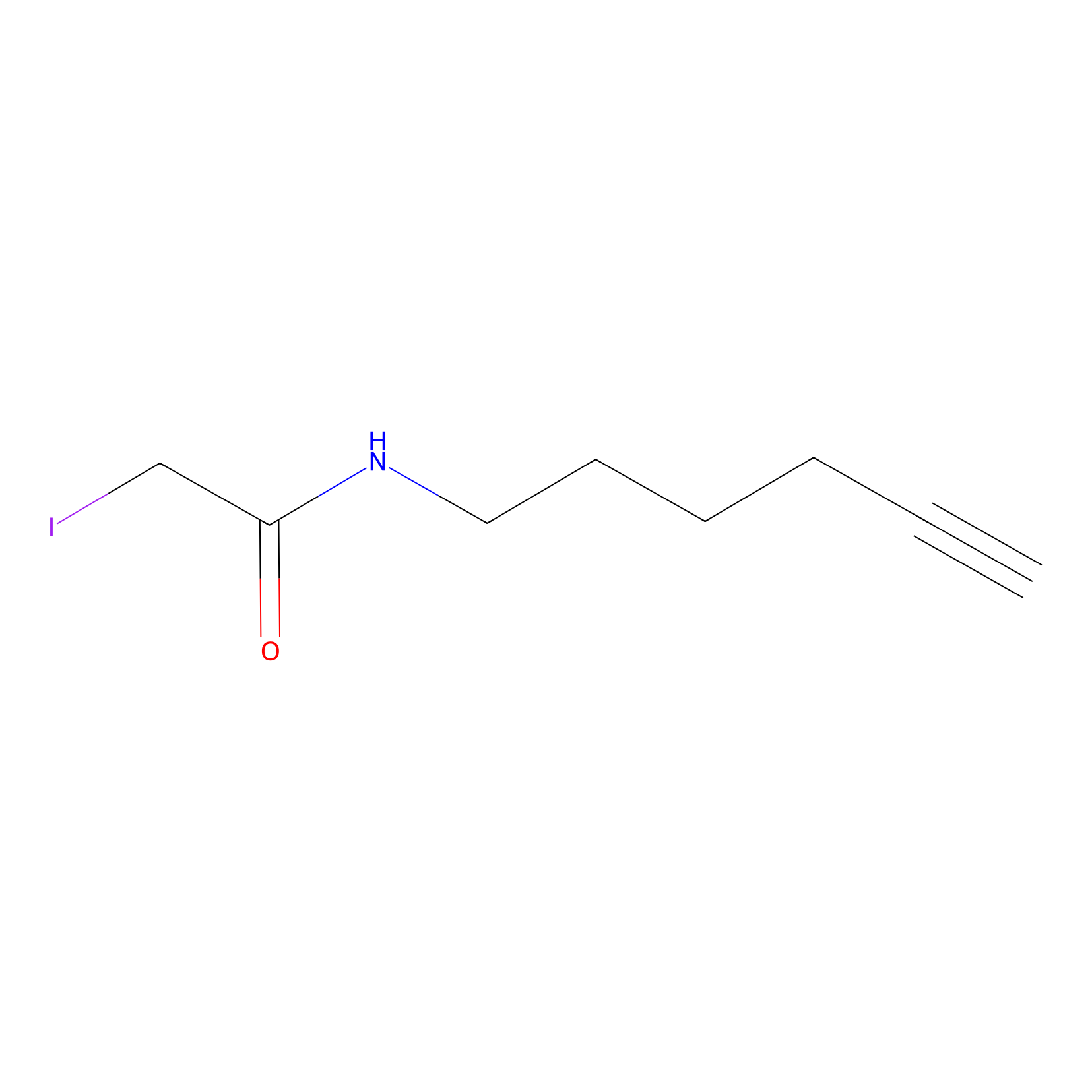 |
C248(0.00); C212(0.00); C311(0.00); C301(0.00) | LDD0036 | [7] | |
|
IPIAA_H Probe Info |
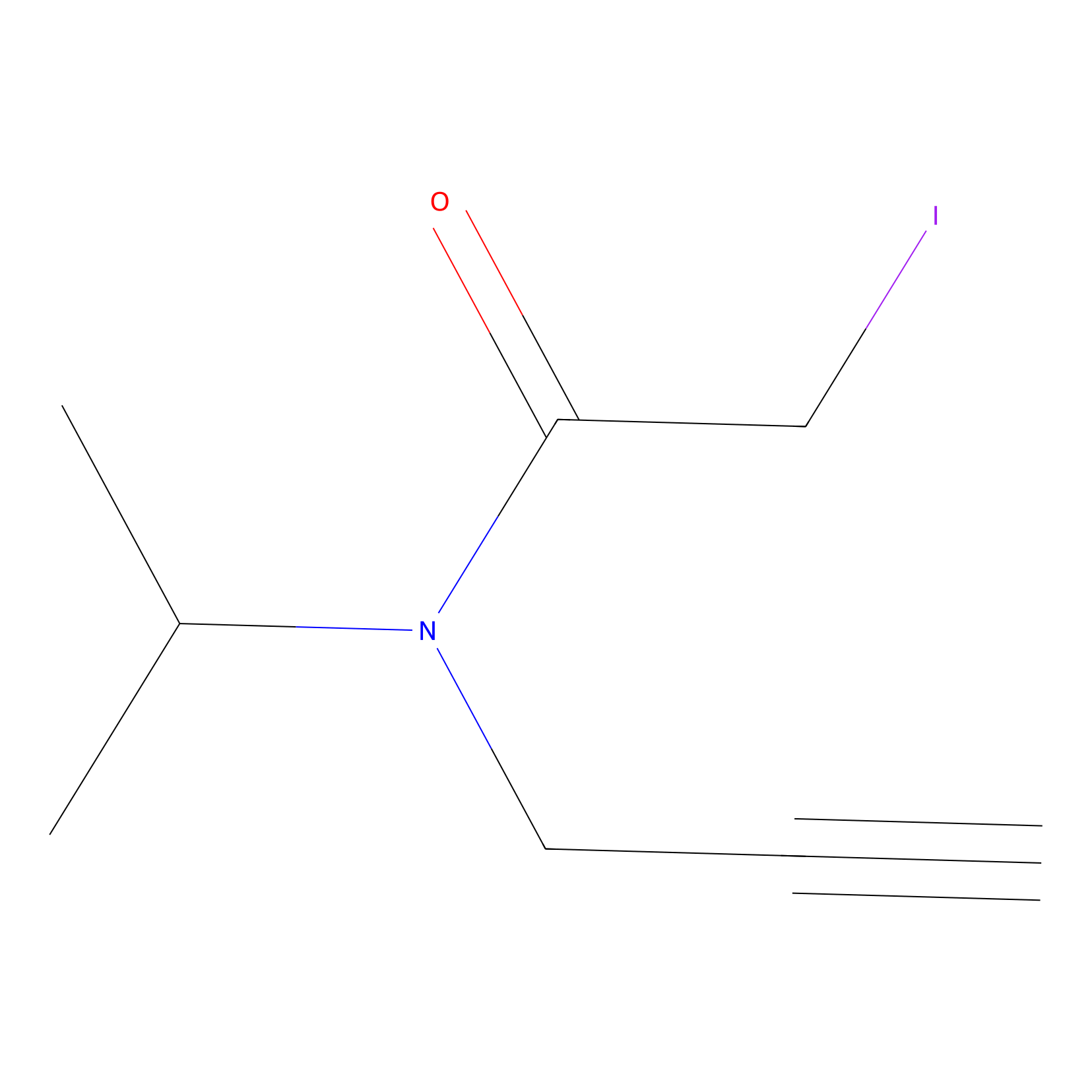 |
N.A. | LDD0030 | [8] | |
|
IPIAA_L Probe Info |
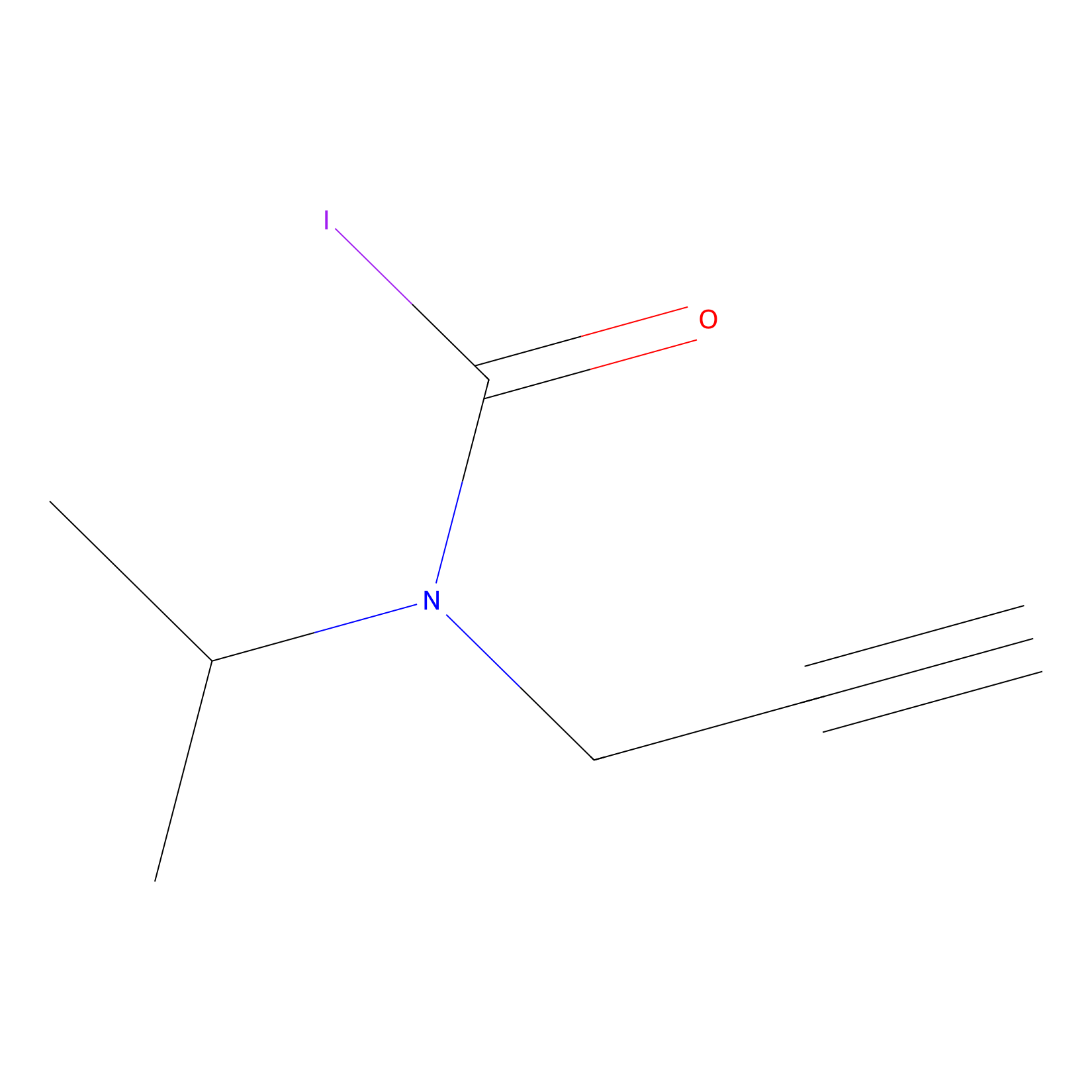 |
N.A. | LDD0031 | [8] | |
|
Lodoacetamide azide Probe Info |
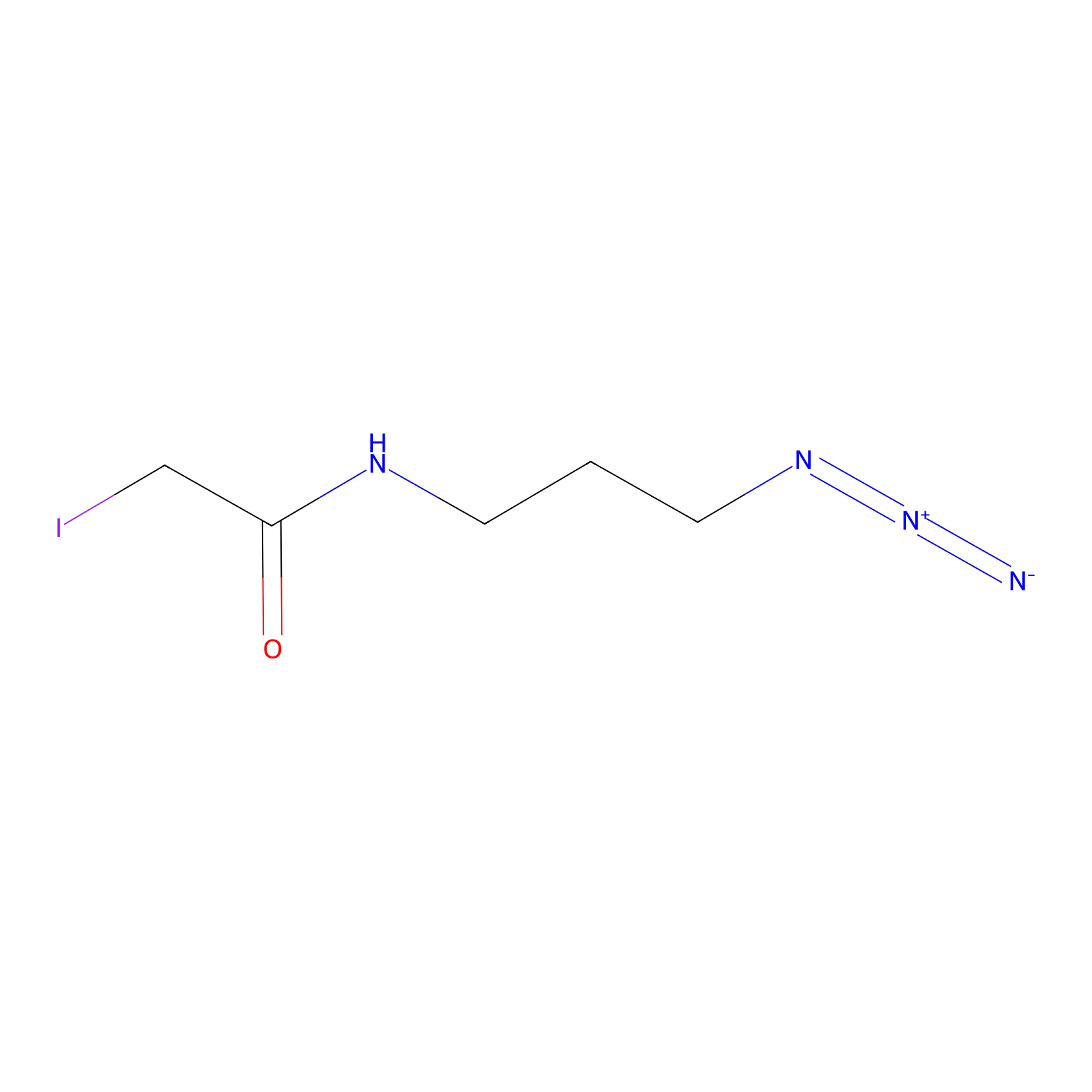 |
C248(0.00); C212(0.00) | LDD0037 | [7] | |
|
NAIA_4 Probe Info |
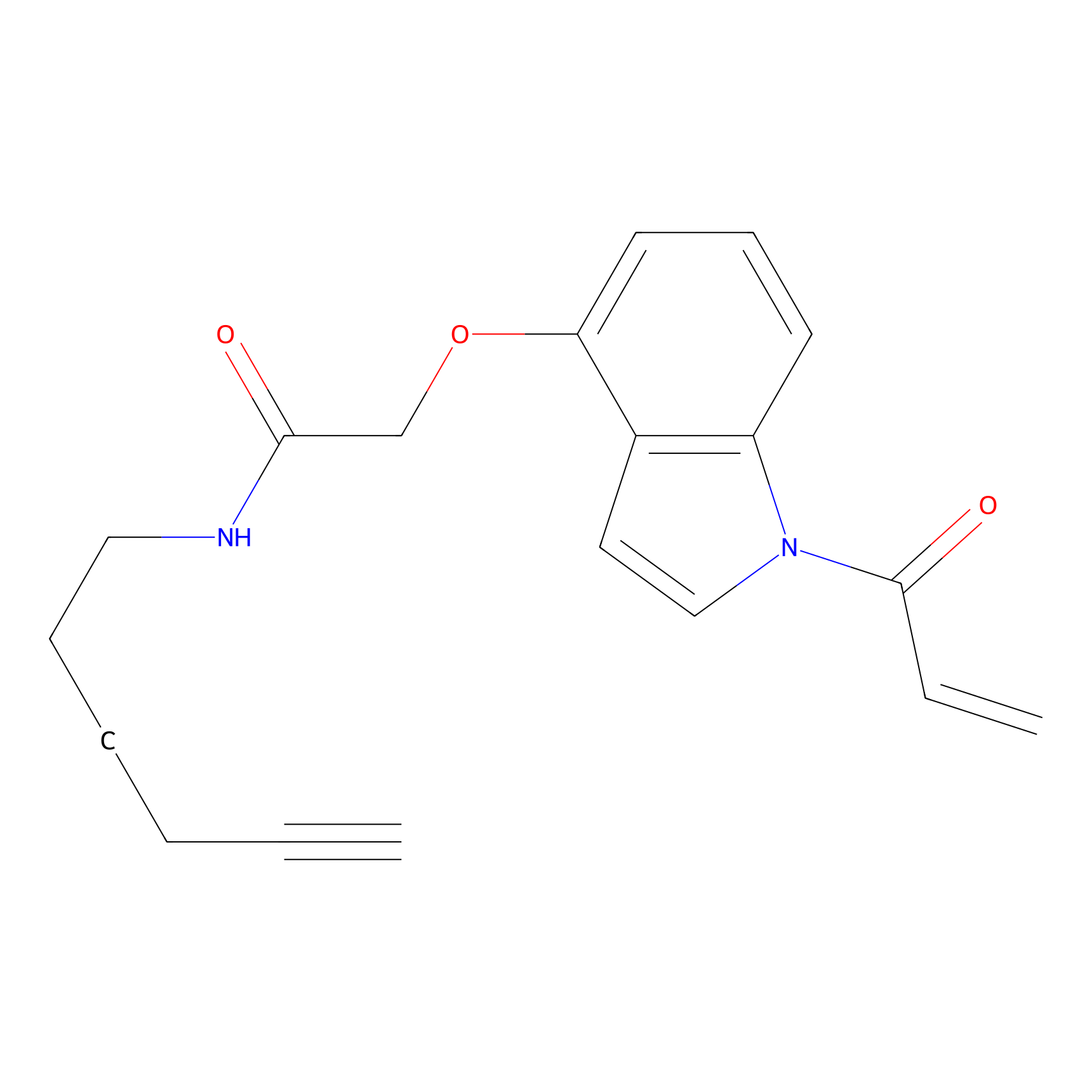 |
N.A. | LDD2226 | [3] | |
|
Compound 10 Probe Info |
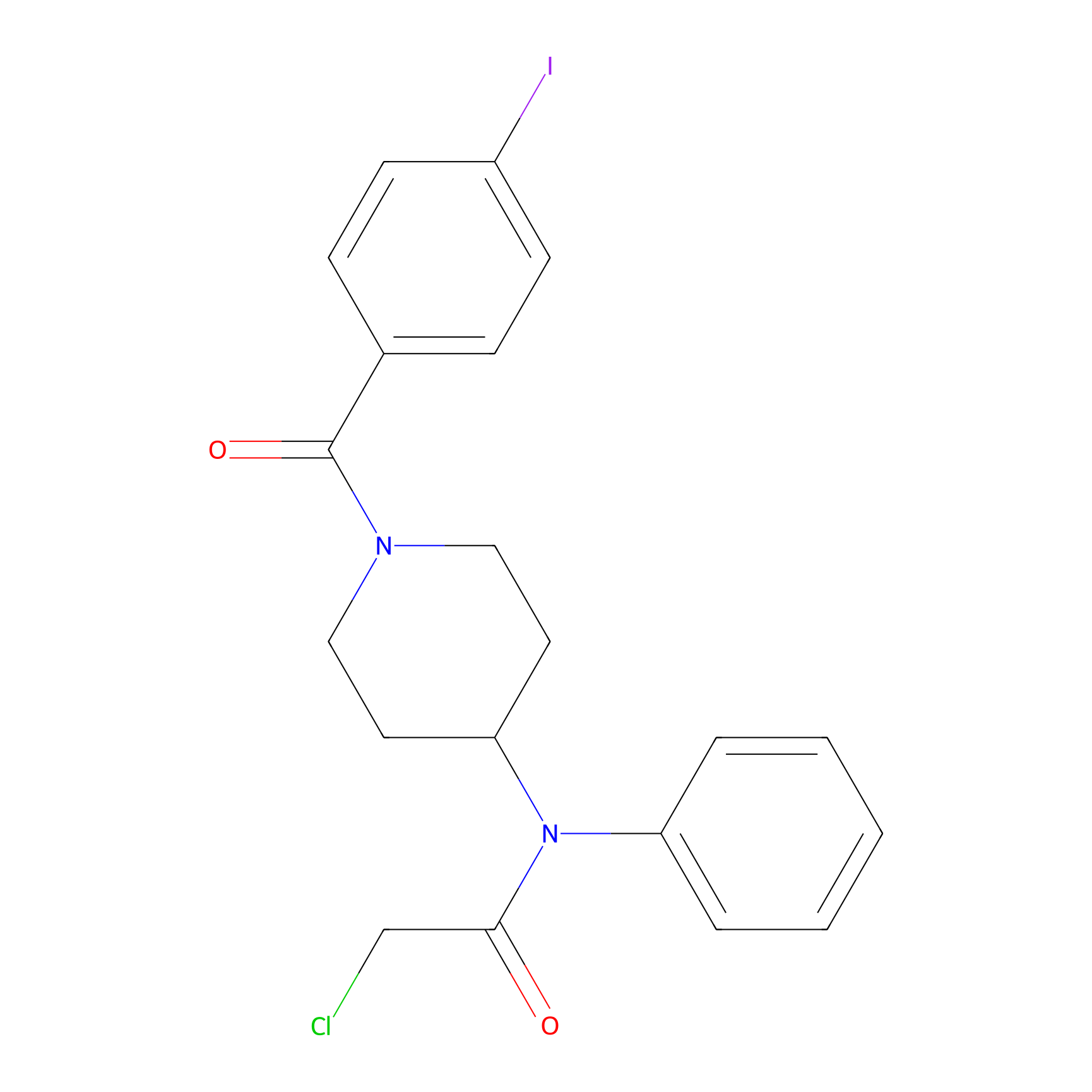 |
N.A. | LDD2216 | [9] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
VE-P Probe Info |
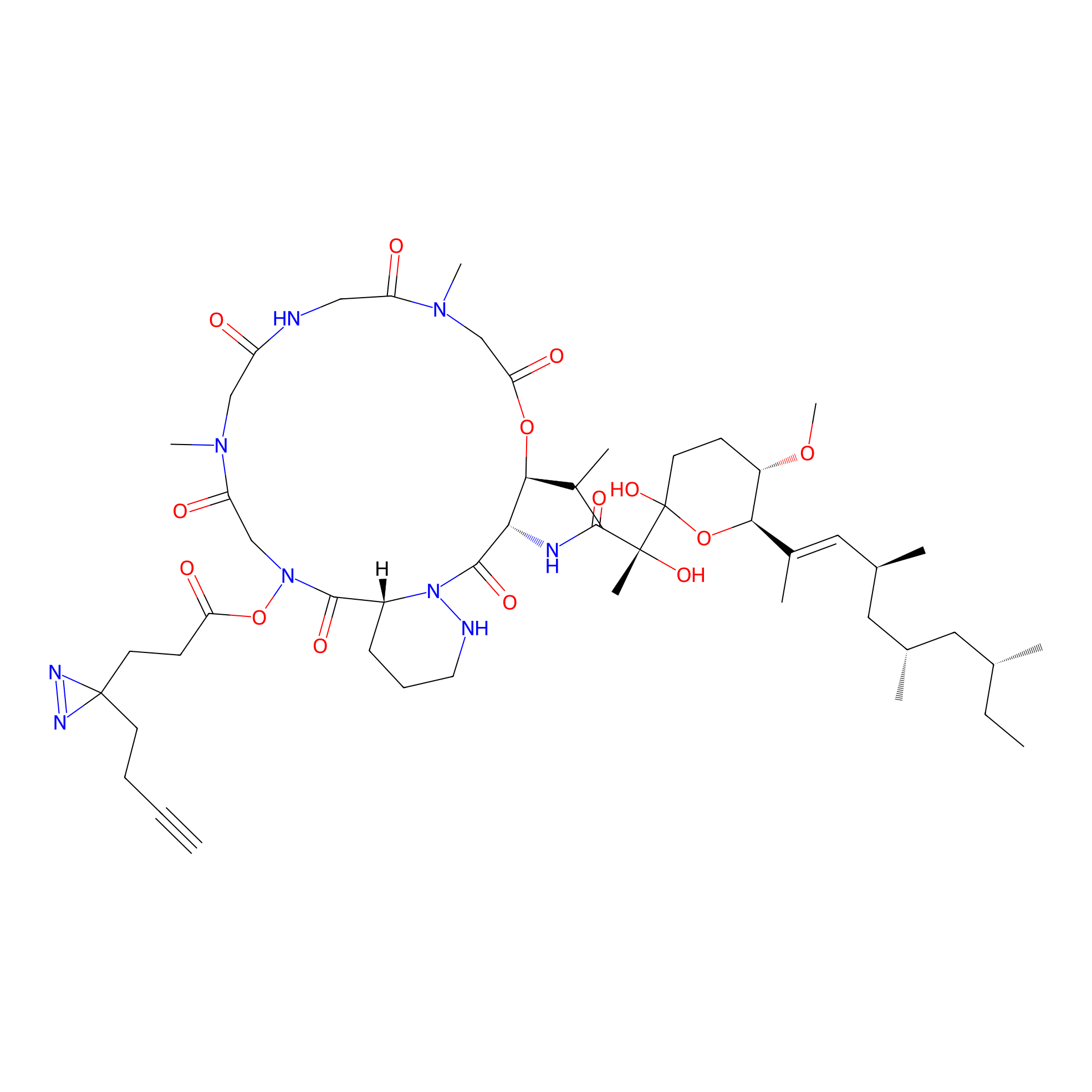 |
N.A. | LDD0396 | [10] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0237 | AC12 | HEK-293T | C248(1.14) | LDD1510 | [11] |
| LDCM0270 | AC15 | HEK-293T | C248(1.05) | LDD1513 | [11] |
| LDCM0280 | AC20 | HEK-293T | C248(1.09) | LDD1519 | [11] |
| LDCM0281 | AC21 | HEK-293T | C248(1.09) | LDD1520 | [11] |
| LDCM0283 | AC23 | HEK-293T | C248(1.05) | LDD1522 | [11] |
| LDCM0288 | AC28 | HEK-293T | C248(1.01) | LDD1527 | [11] |
| LDCM0289 | AC29 | HEK-293T | C248(0.98) | LDD1528 | [11] |
| LDCM0292 | AC31 | HEK-293T | C248(1.14) | LDD1531 | [11] |
| LDCM0297 | AC36 | HEK-293T | C248(1.11) | LDD1536 | [11] |
| LDCM0298 | AC37 | HEK-293T | C248(1.11) | LDD1537 | [11] |
| LDCM0300 | AC39 | HEK-293T | C248(1.15) | LDD1539 | [11] |
| LDCM0301 | AC4 | HEK-293T | C248(1.18) | LDD1540 | [11] |
| LDCM0306 | AC44 | HEK-293T | C248(1.24) | LDD1545 | [11] |
| LDCM0307 | AC45 | HEK-293T | C248(0.97) | LDD1546 | [11] |
| LDCM0309 | AC47 | HEK-293T | C248(1.16) | LDD1548 | [11] |
| LDCM0312 | AC5 | HEK-293T | C248(1.06) | LDD1551 | [11] |
| LDCM0315 | AC52 | HEK-293T | C248(1.22) | LDD1554 | [11] |
| LDCM0316 | AC53 | HEK-293T | C248(1.06) | LDD1555 | [11] |
| LDCM0318 | AC55 | HEK-293T | C248(0.99) | LDD1557 | [11] |
| LDCM0324 | AC60 | HEK-293T | C248(0.99) | LDD1563 | [11] |
| LDCM0325 | AC61 | HEK-293T | C248(1.02) | LDD1564 | [11] |
| LDCM0327 | AC63 | HEK-293T | C248(1.08) | LDD1566 | [11] |
| LDCM0334 | AC7 | HEK-293T | C248(1.14) | LDD1568 | [11] |
| LDCM0248 | AKOS034007472 | HEK-293T | C248(1.03) | LDD1511 | [11] |
| LDCM0632 | CL-Sc | Hep-G2 | C248(20.00) | LDD2227 | [3] |
| LDCM0379 | CL11 | HEK-293T | C248(0.96) | LDD1583 | [11] |
| LDCM0408 | CL20 | HEK-293T | C248(1.04) | LDD1612 | [11] |
| LDCM0409 | CL21 | HEK-293T | C248(0.78) | LDD1613 | [11] |
| LDCM0411 | CL23 | HEK-293T | C248(0.89) | LDD1615 | [11] |
| LDCM0421 | CL32 | HEK-293T | C248(0.94) | LDD1625 | [11] |
| LDCM0422 | CL33 | HEK-293T | C248(0.97) | LDD1626 | [11] |
| LDCM0424 | CL35 | HEK-293T | C248(0.89) | LDD1628 | [11] |
| LDCM0434 | CL44 | HEK-293T | C248(0.91) | LDD1638 | [11] |
| LDCM0435 | CL45 | HEK-293T | C248(0.87) | LDD1639 | [11] |
| LDCM0437 | CL47 | HEK-293T | C248(0.88) | LDD1641 | [11] |
| LDCM0447 | CL56 | HEK-293T | C248(1.12) | LDD1650 | [11] |
| LDCM0448 | CL57 | HEK-293T | C248(0.94) | LDD1651 | [11] |
| LDCM0450 | CL59 | HEK-293T | C248(0.96) | LDD1653 | [11] |
| LDCM0460 | CL68 | HEK-293T | C248(1.00) | LDD1663 | [11] |
| LDCM0461 | CL69 | HEK-293T | C248(0.98) | LDD1664 | [11] |
| LDCM0464 | CL71 | HEK-293T | C248(0.88) | LDD1667 | [11] |
| LDCM0473 | CL8 | HEK-293T | C248(1.16) | LDD1676 | [11] |
| LDCM0474 | CL80 | HEK-293T | C248(1.00) | LDD1677 | [11] |
| LDCM0475 | CL81 | HEK-293T | C248(0.97) | LDD1678 | [11] |
| LDCM0477 | CL83 | HEK-293T | C248(0.87) | LDD1680 | [11] |
| LDCM0484 | CL9 | HEK-293T | C248(1.03) | LDD1687 | [11] |
| LDCM0487 | CL92 | HEK-293T | C248(1.04) | LDD1690 | [11] |
| LDCM0488 | CL93 | HEK-293T | C248(1.06) | LDD1691 | [11] |
| LDCM0490 | CL95 | HEK-293T | C248(0.82) | LDD1693 | [11] |
| LDCM0625 | F8 | Ramos | C248(1.28) | LDD2187 | [12] |
| LDCM0572 | Fragment10 | Ramos | C248(1.84) | LDD2189 | [12] |
| LDCM0573 | Fragment11 | Ramos | C248(0.05) | LDD2190 | [12] |
| LDCM0574 | Fragment12 | Ramos | C248(1.60) | LDD2191 | [12] |
| LDCM0575 | Fragment13 | Ramos | C248(1.24) | LDD2192 | [12] |
| LDCM0576 | Fragment14 | Ramos | C248(0.88) | LDD2193 | [12] |
| LDCM0579 | Fragment20 | Ramos | C248(1.33) | LDD2194 | [12] |
| LDCM0580 | Fragment21 | Ramos | C248(1.03) | LDD2195 | [12] |
| LDCM0582 | Fragment23 | Ramos | C248(1.21) | LDD2196 | [12] |
| LDCM0578 | Fragment27 | Ramos | C248(0.97) | LDD2197 | [12] |
| LDCM0586 | Fragment28 | Ramos | C248(0.93) | LDD2198 | [12] |
| LDCM0588 | Fragment30 | Ramos | C248(1.19) | LDD2199 | [12] |
| LDCM0589 | Fragment31 | Ramos | C248(1.55) | LDD2200 | [12] |
| LDCM0590 | Fragment32 | Ramos | C248(1.64) | LDD2201 | [12] |
| LDCM0468 | Fragment33 | Ramos | C248(2.93) | LDD2202 | [12] |
| LDCM0596 | Fragment38 | Ramos | C248(1.00) | LDD2203 | [12] |
| LDCM0566 | Fragment4 | Ramos | C248(2.05) | LDD2184 | [12] |
| LDCM0610 | Fragment52 | Ramos | C248(1.45) | LDD2204 | [12] |
| LDCM0614 | Fragment56 | Ramos | C248(0.97) | LDD2205 | [12] |
| LDCM0569 | Fragment7 | Ramos | C248(2.21) | LDD2186 | [12] |
| LDCM0571 | Fragment9 | Ramos | C248(1.55) | LDD2188 | [12] |
| LDCM0116 | HHS-0101 | DM93 | Y360(1.51) | LDD0264 | [6] |
| LDCM0117 | HHS-0201 | DM93 | Y360(1.32) | LDD0265 | [6] |
| LDCM0118 | HHS-0301 | DM93 | Y360(0.71) | LDD0266 | [6] |
| LDCM0119 | HHS-0401 | DM93 | Y360(0.67) | LDD0267 | [6] |
| LDCM0120 | HHS-0701 | DM93 | Y360(0.86) | LDD0268 | [6] |
| LDCM0123 | JWB131 | DM93 | Y360(1.02) | LDD0285 | [5] |
| LDCM0124 | JWB142 | DM93 | Y360(0.66) | LDD0286 | [5] |
| LDCM0125 | JWB146 | DM93 | Y360(0.79) | LDD0287 | [5] |
| LDCM0126 | JWB150 | DM93 | Y360(3.43) | LDD0288 | [5] |
| LDCM0127 | JWB152 | DM93 | Y360(2.54) | LDD0289 | [5] |
| LDCM0128 | JWB198 | DM93 | Y360(1.30) | LDD0290 | [5] |
| LDCM0129 | JWB202 | DM93 | Y360(0.86) | LDD0291 | [5] |
| LDCM0130 | JWB211 | DM93 | Y360(1.11) | LDD0292 | [5] |
| LDCM0022 | KB02 | HEK-293T | C248(0.99) | LDD1492 | [11] |
| LDCM0023 | KB03 | Jurkat | C248(27.72) | LDD0209 | [4] |
| LDCM0024 | KB05 | COLO792 | C296(1.35) | LDD3310 | [13] |
| LDCM0131 | RA190 | MM1.R | C248(1.36) | LDD0304 | [14] |
The Interaction Atlas With This Target
References
