Details of the Target
General Information of Target
| Target ID | LDTP04109 | |||||
|---|---|---|---|---|---|---|
| Target Name | Small ribosomal subunit protein uS4 (RPS9) | |||||
| Gene Name | RPS9 | |||||
| Gene ID | 6203 | |||||
| Synonyms |
Small ribosomal subunit protein uS4; 40S ribosomal protein S9 |
|||||
| 3D Structure | ||||||
| Sequence |
MPVARSWVCRKTYVTPRRPFEKSRLDQELKLIGEYGLRNKREVWRVKFTLAKIRKAAREL
LTLDEKDPRRLFEGNALLRRLVRIGVLDEGKMKLDYILGLKIEDFLERRLQTQVFKLGLA KSIHHARVLIRQRHIRVRKQVVNIPSFIVRLDSQKHIDFSLRSPYGGGRPGRVKRKNAKK GQGGAGAGDDEEED |
|||||
| Target Bioclass |
Other
|
|||||
| Family |
Universal ribosomal protein uS4 family
|
|||||
| Subcellular location |
Cytoplasm
|
|||||
| Function |
Component of the small ribosomal subunit. The ribosome is a large ribonucleoprotein complex responsible for the synthesis of proteins in the cell. Part of the small subunit (SSU) processome, first precursor of the small eukaryotic ribosomal subunit. During the assembly of the SSU processome in the nucleolus, many ribosome biogenesis factors, an RNA chaperone and ribosomal proteins associate with the nascent pre-rRNA and work in concert to generate RNA folding, modifications, rearrangements and cleavage as well as targeted degradation of pre-ribosomal RNA by the RNA exosome.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
m-APA Probe Info |
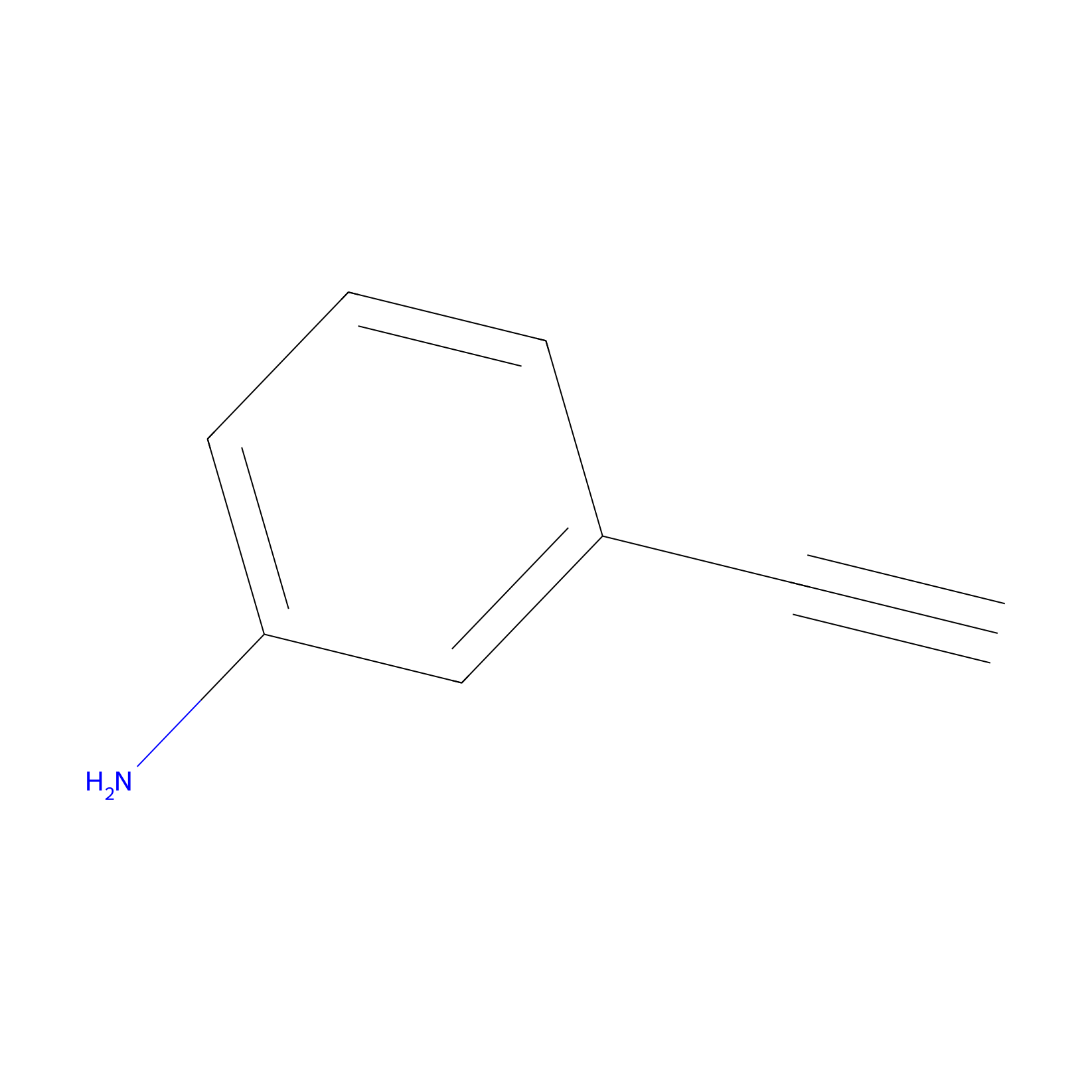 |
13.01 | LDD0402 | [1] | |
|
A-EBA Probe Info |
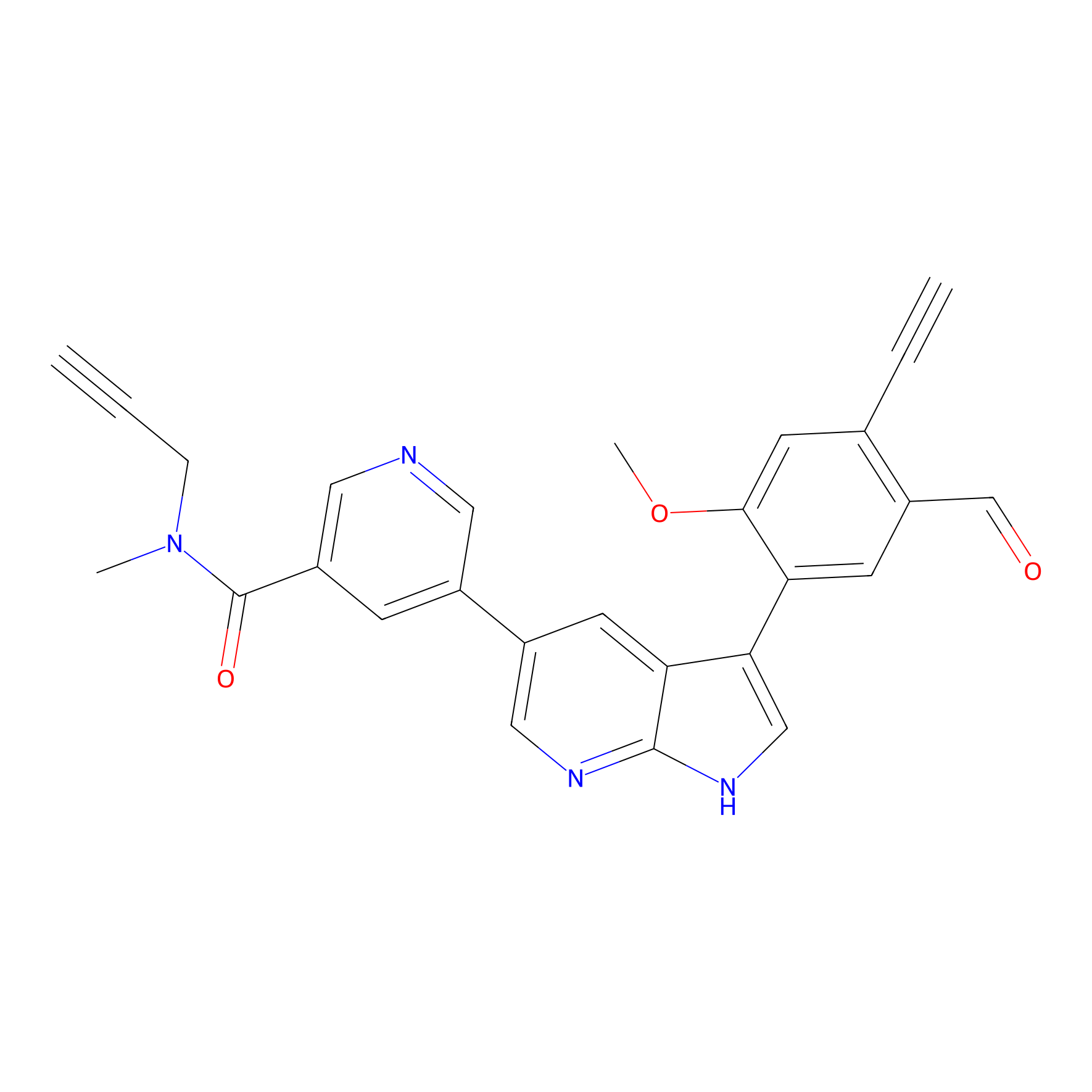 |
3.43 | LDD0215 | [2] | |
|
CHEMBL5175495 Probe Info |
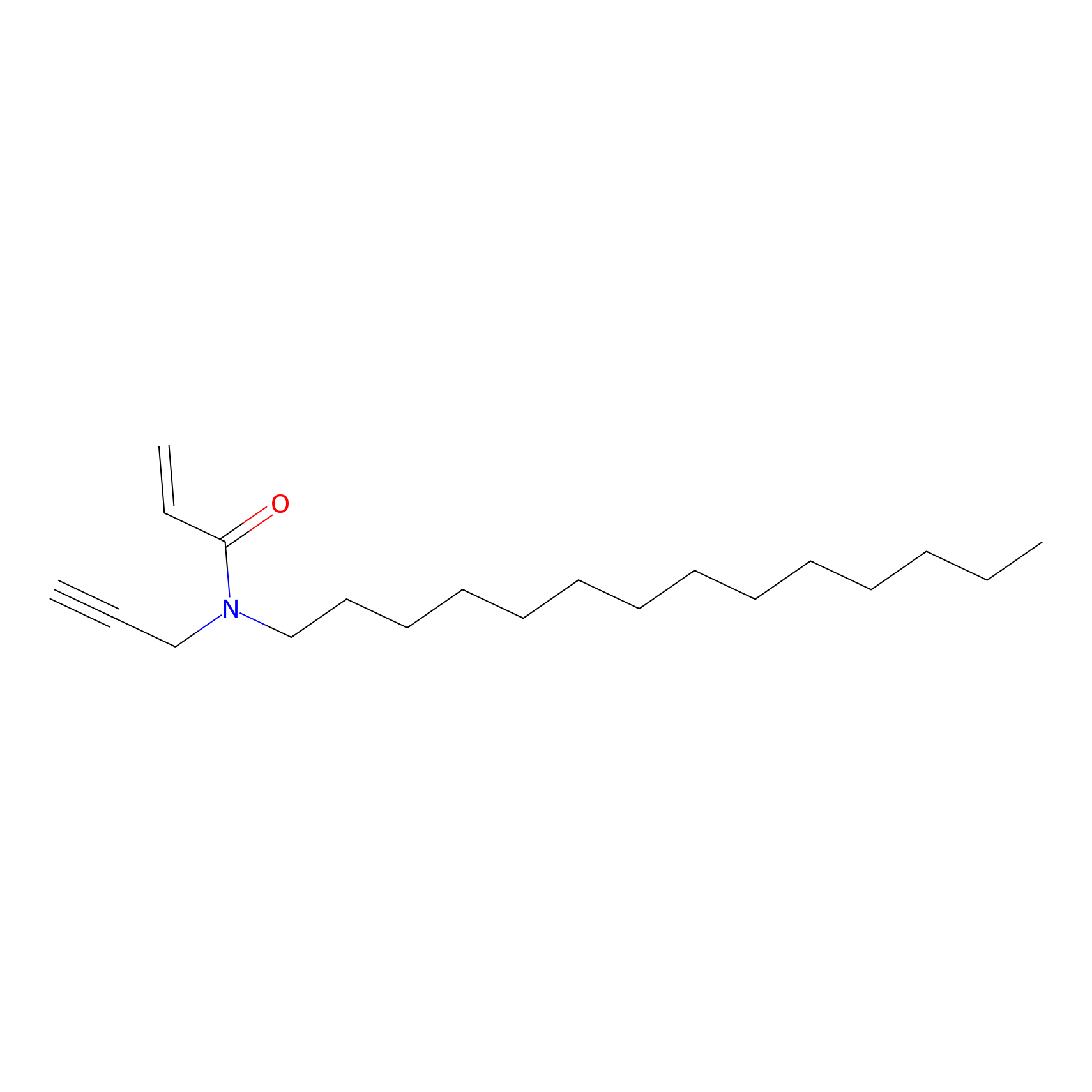 |
6.56 | LDD0196 | [3] | |
|
C-Sul Probe Info |
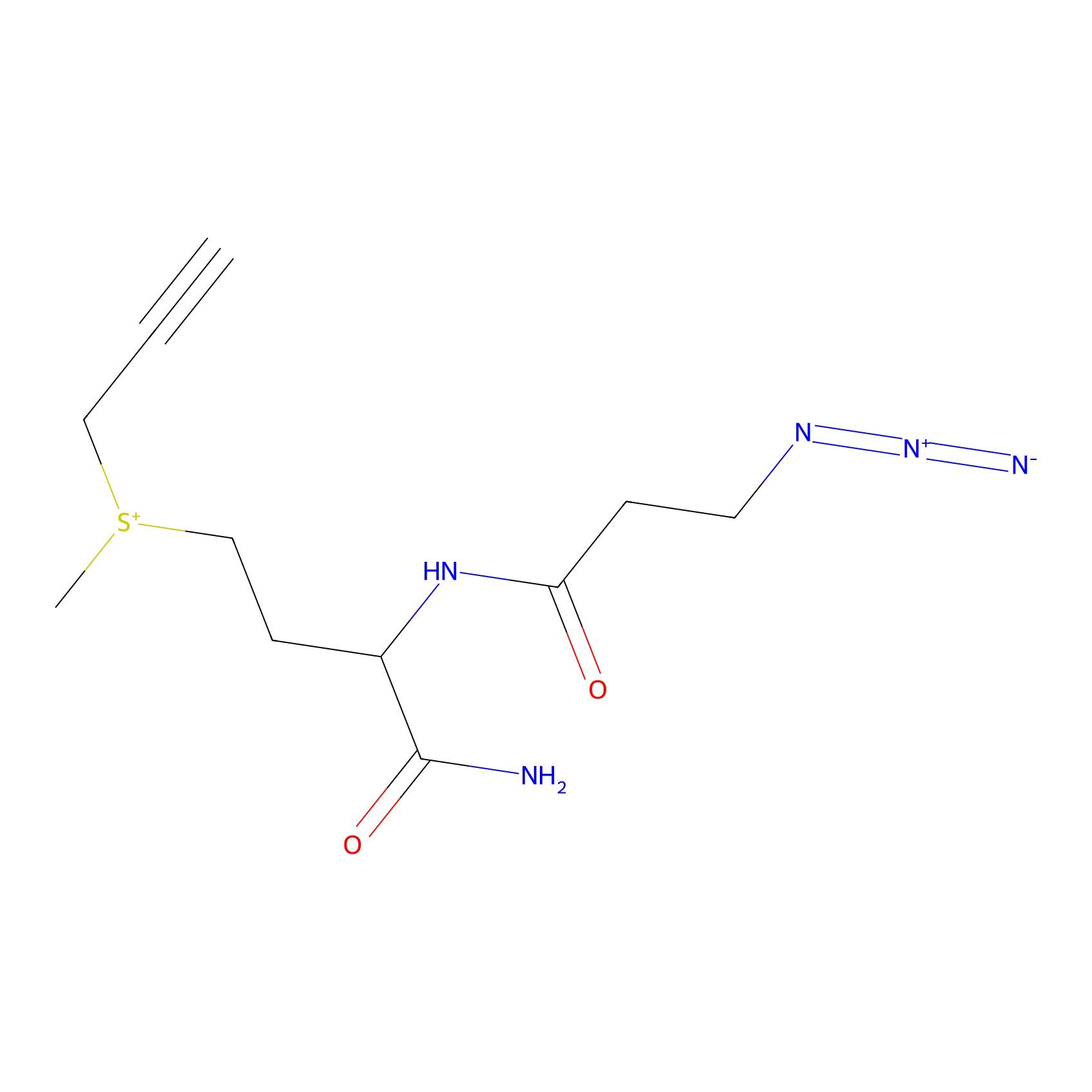 |
3.43 | LDD0066 | [4] | |
|
TH211 Probe Info |
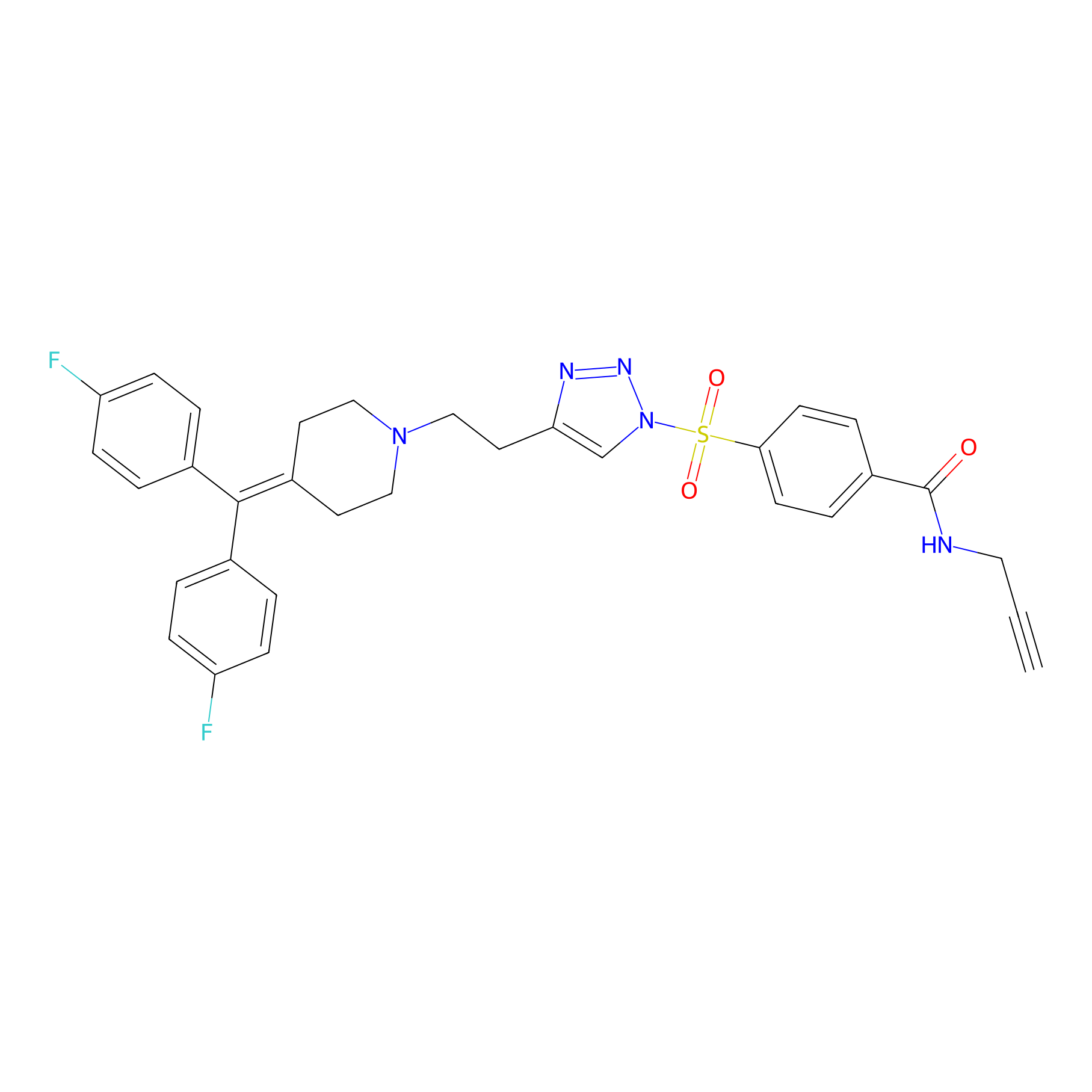 |
Y13(20.00); Y165(20.00); Y96(11.89) | LDD0257 | [5] | |
|
TH214 Probe Info |
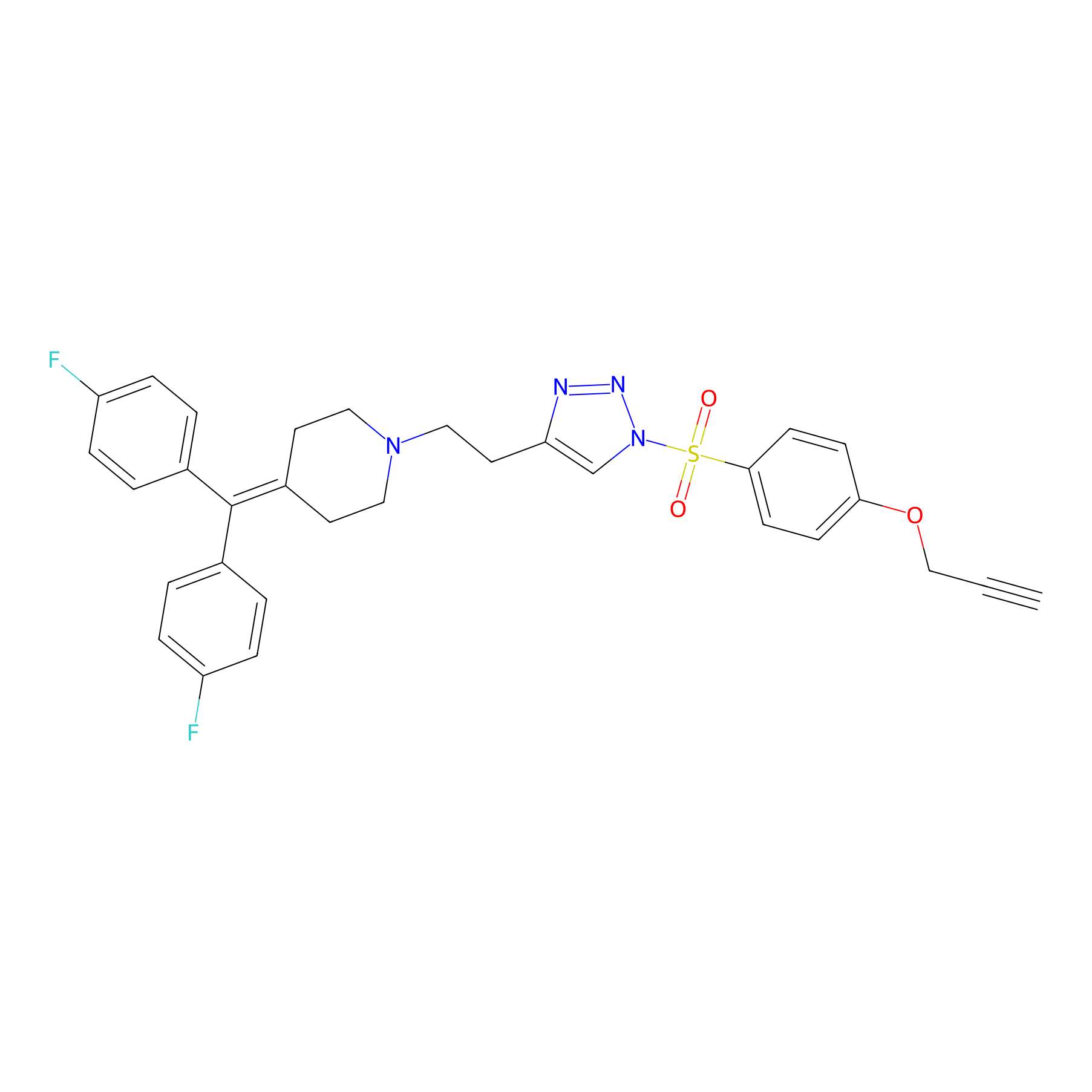 |
Y13(12.50) | LDD0258 | [5] | |
|
TH216 Probe Info |
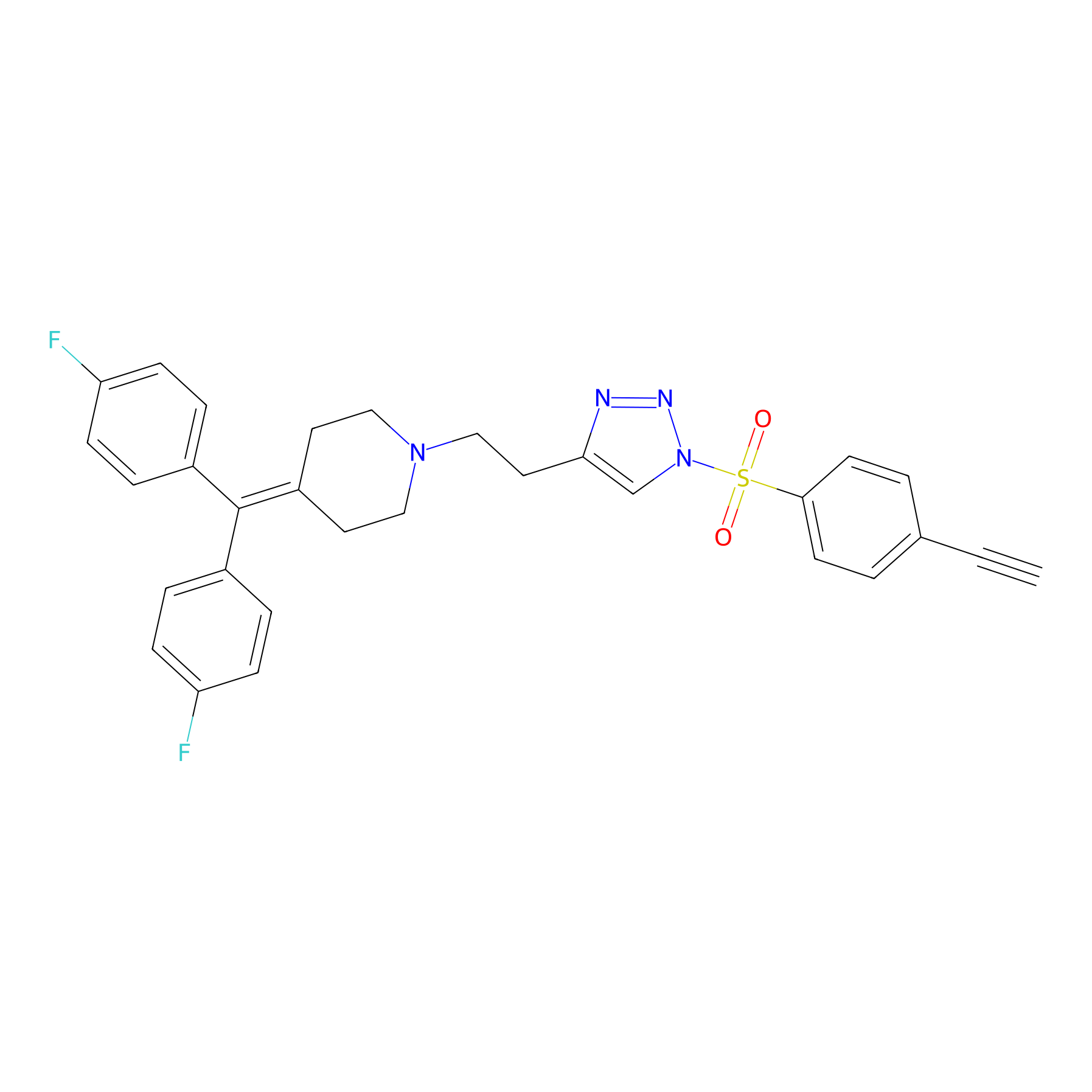 |
Y96(17.90); Y35(11.53); Y13(11.11) | LDD0259 | [5] | |
|
YN-4 Probe Info |
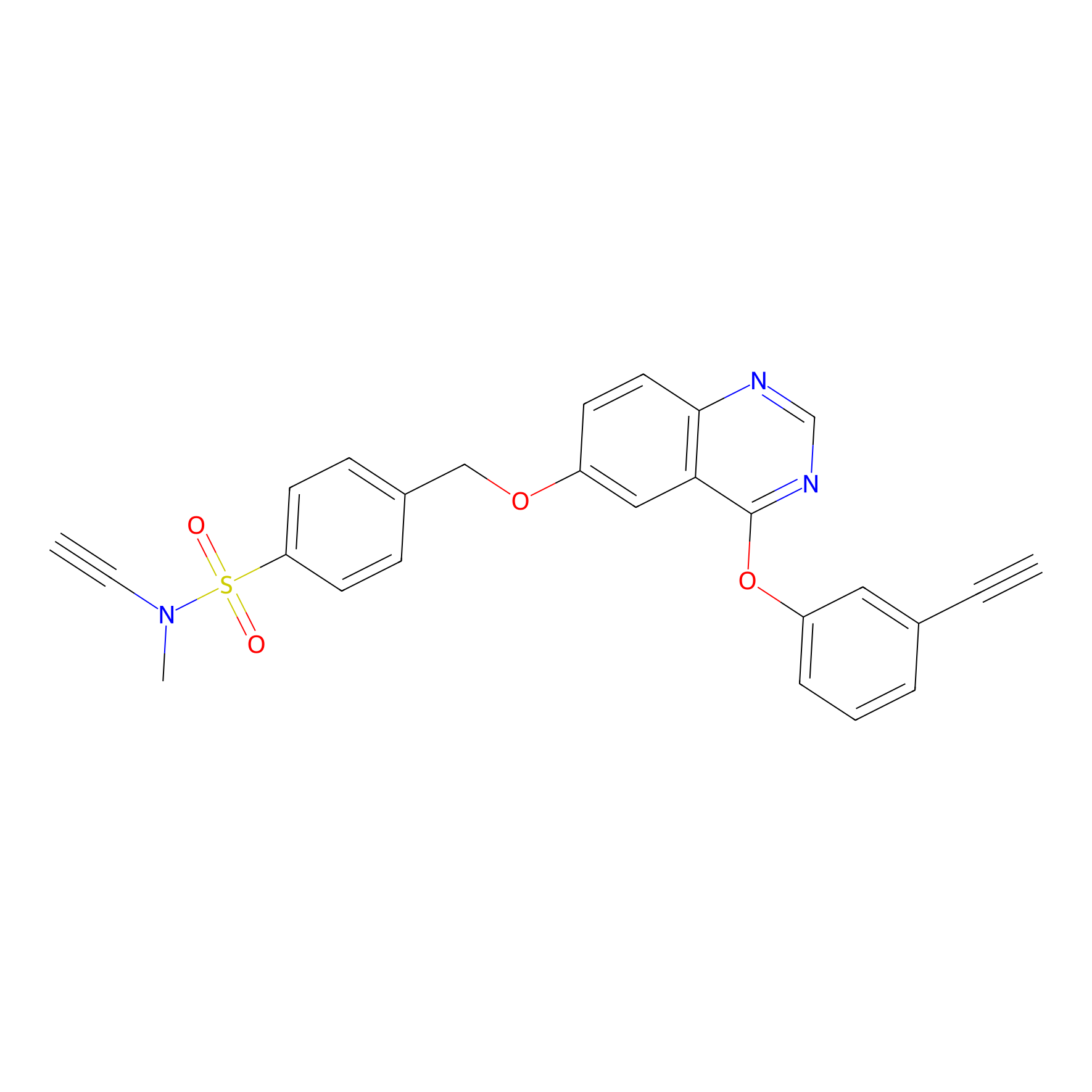 |
100.00 | LDD0445 | [6] | |
|
ONAyne Probe Info |
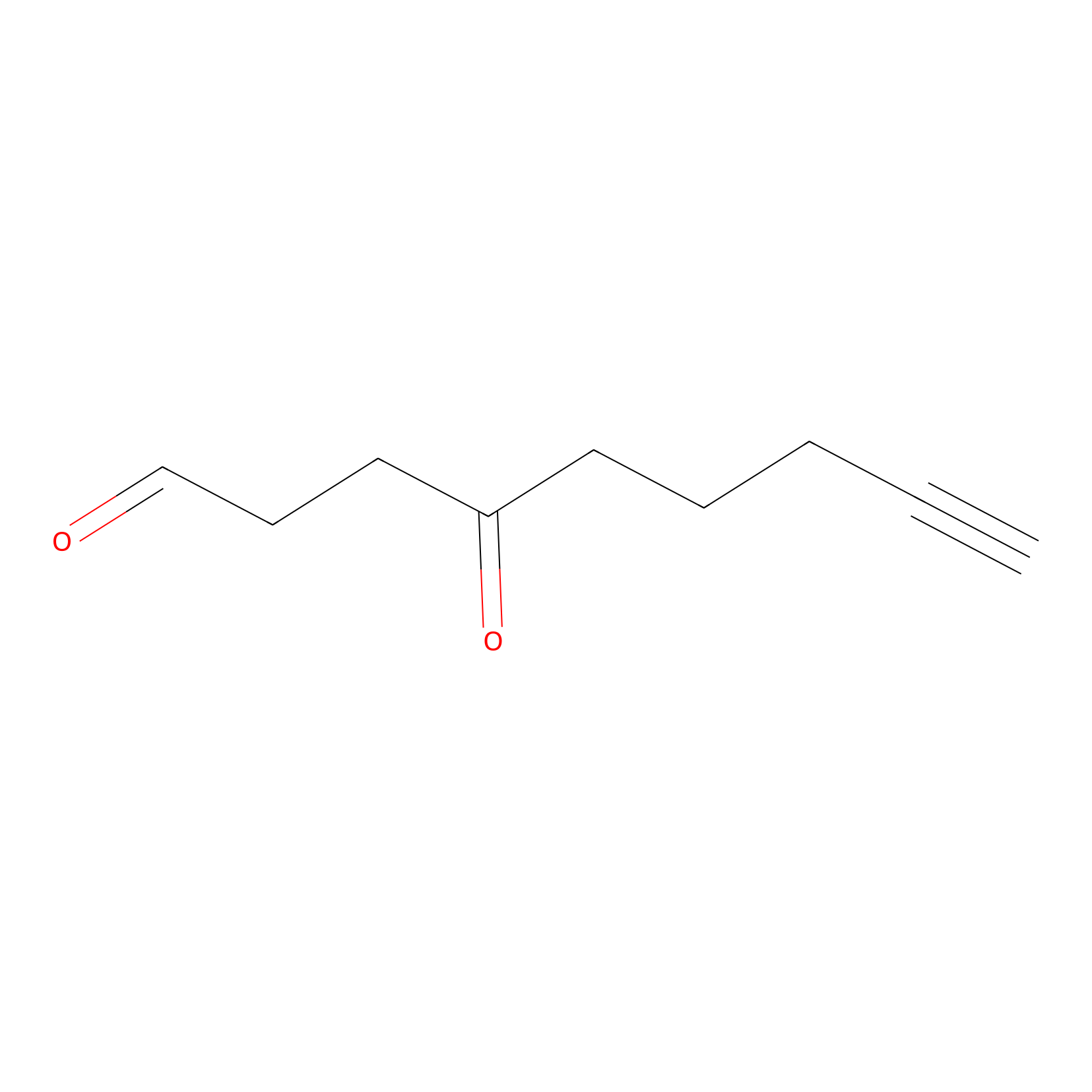 |
K11(0.00); K22(0.00); K30(0.00); K155(0.00) | LDD0273 | [7] | |
|
OPA-S-S-alkyne Probe Info |
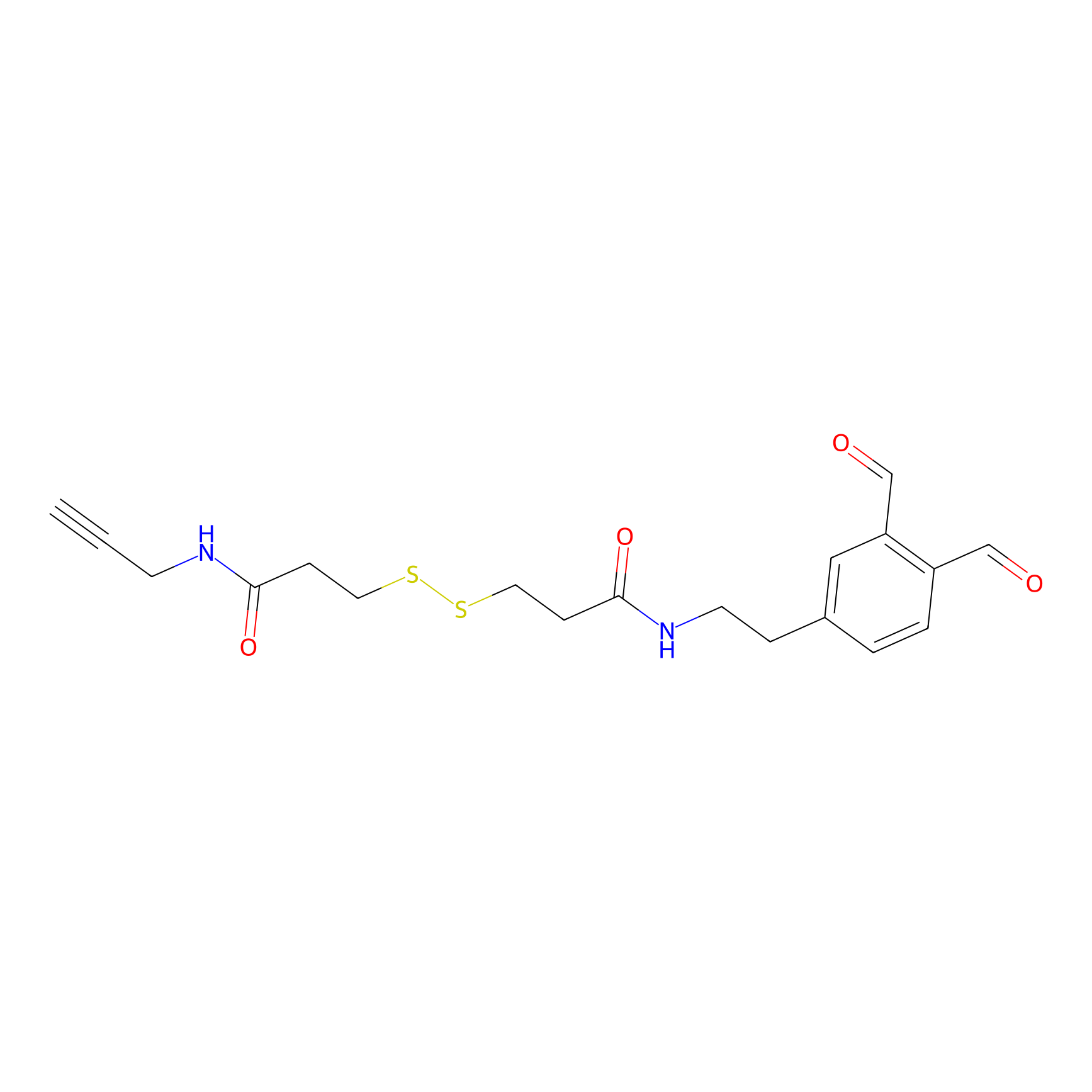 |
K30(1.01); K155(2.28); K52(2.39); K66(2.63) | LDD3494 | [8] | |
|
Probe 1 Probe Info |
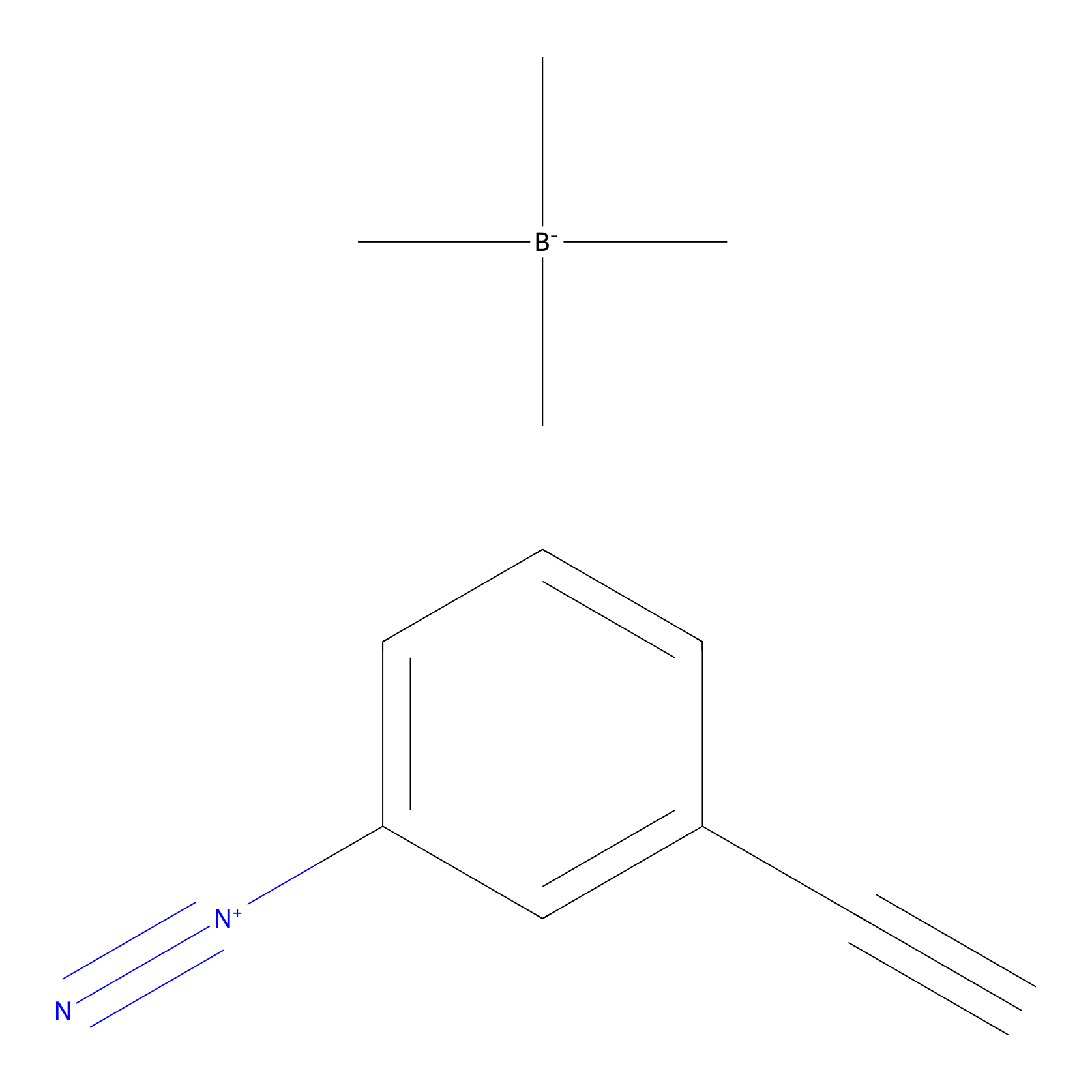 |
Y35(11.99); Y165(584,252.31) | LDD3495 | [9] | |
|
DBIA Probe Info |
 |
C169(1.61) | LDD3432 | [10] | |
|
AZ-9 Probe Info |
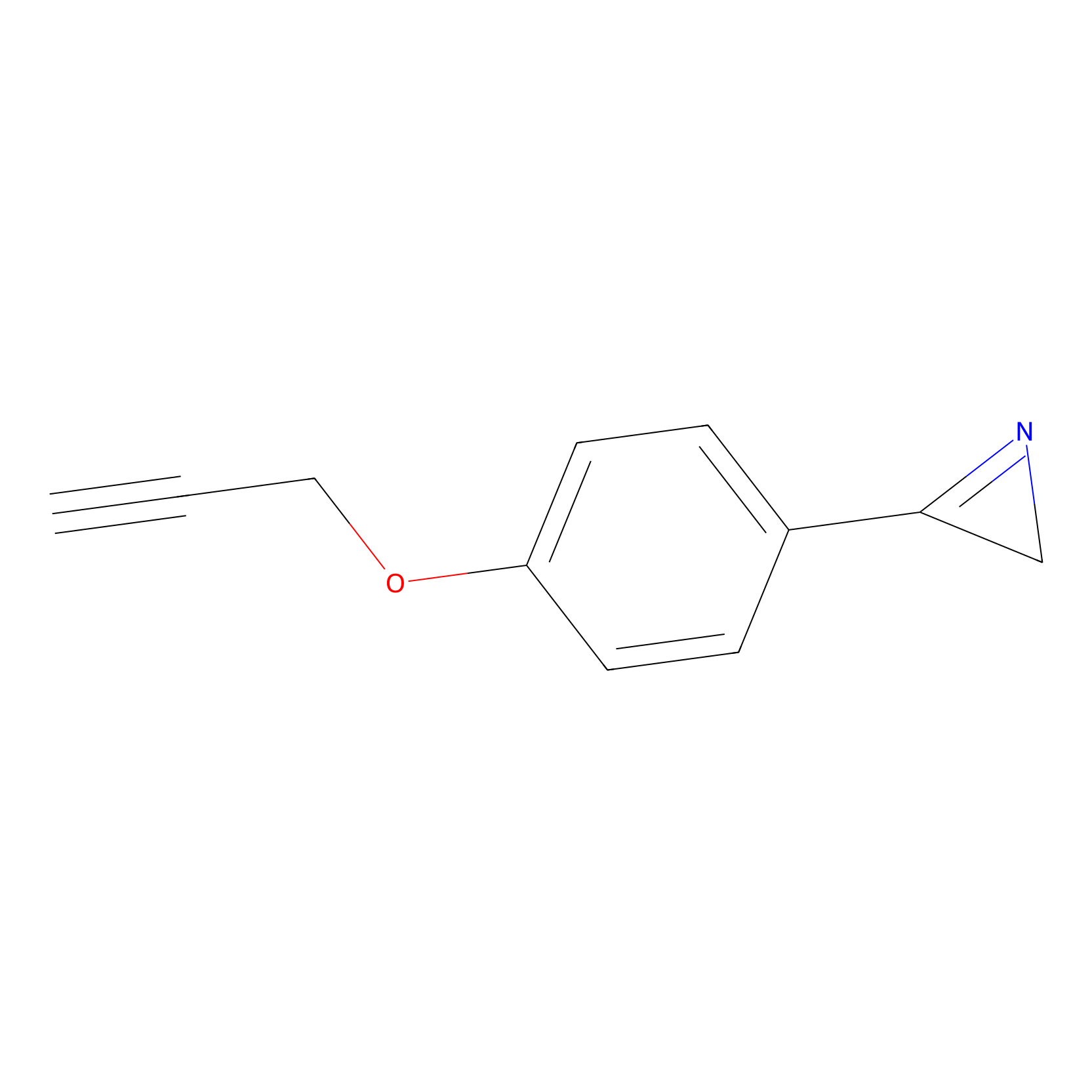 |
10.00 | LDD2154 | [11] | |
|
HHS-475 Probe Info |
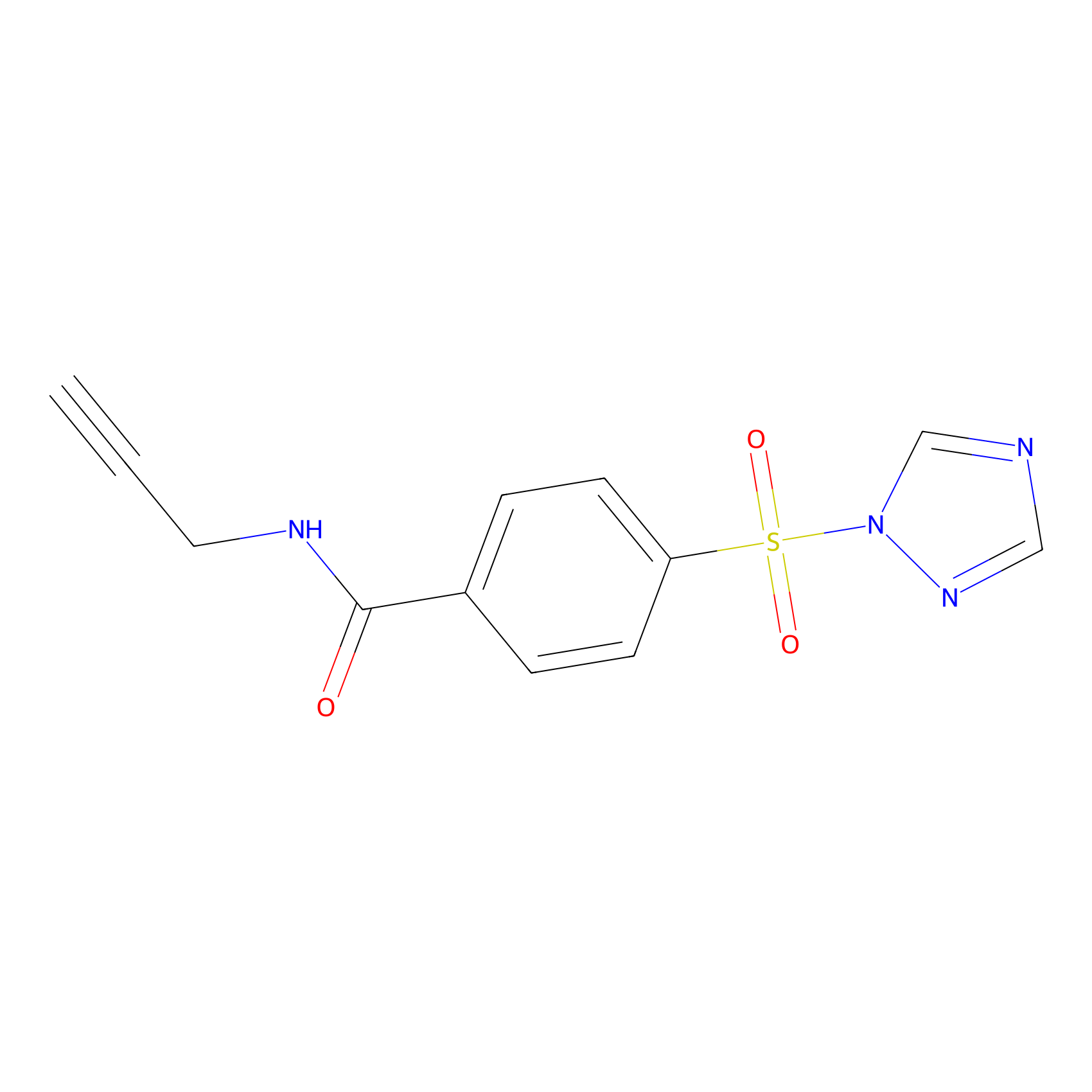 |
Y96(0.65) | LDD0264 | [12] | |
|
HHS-465 Probe Info |
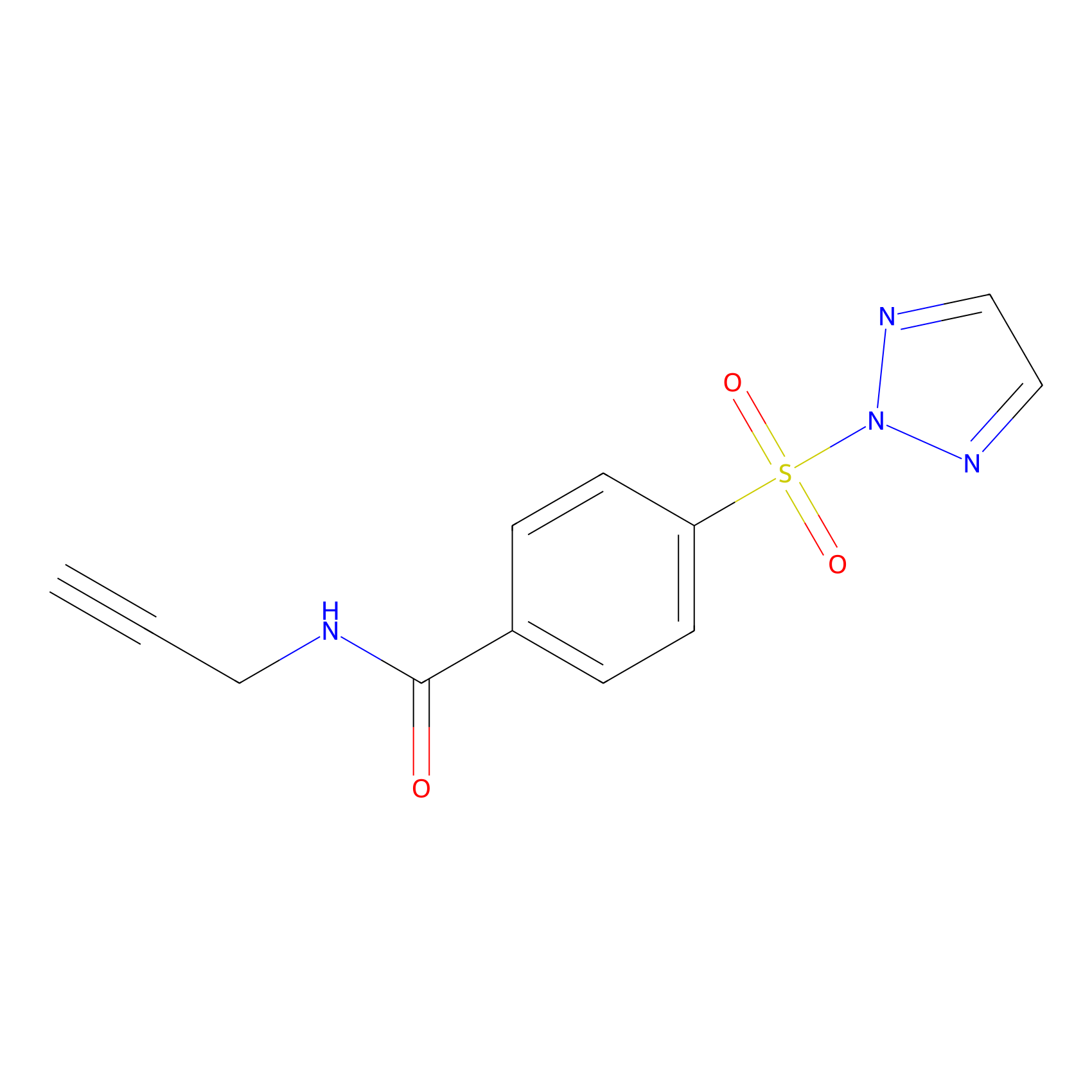 |
Y13(6.27); Y96(10.00) | LDD2237 | [13] | |
|
Acrolein Probe Info |
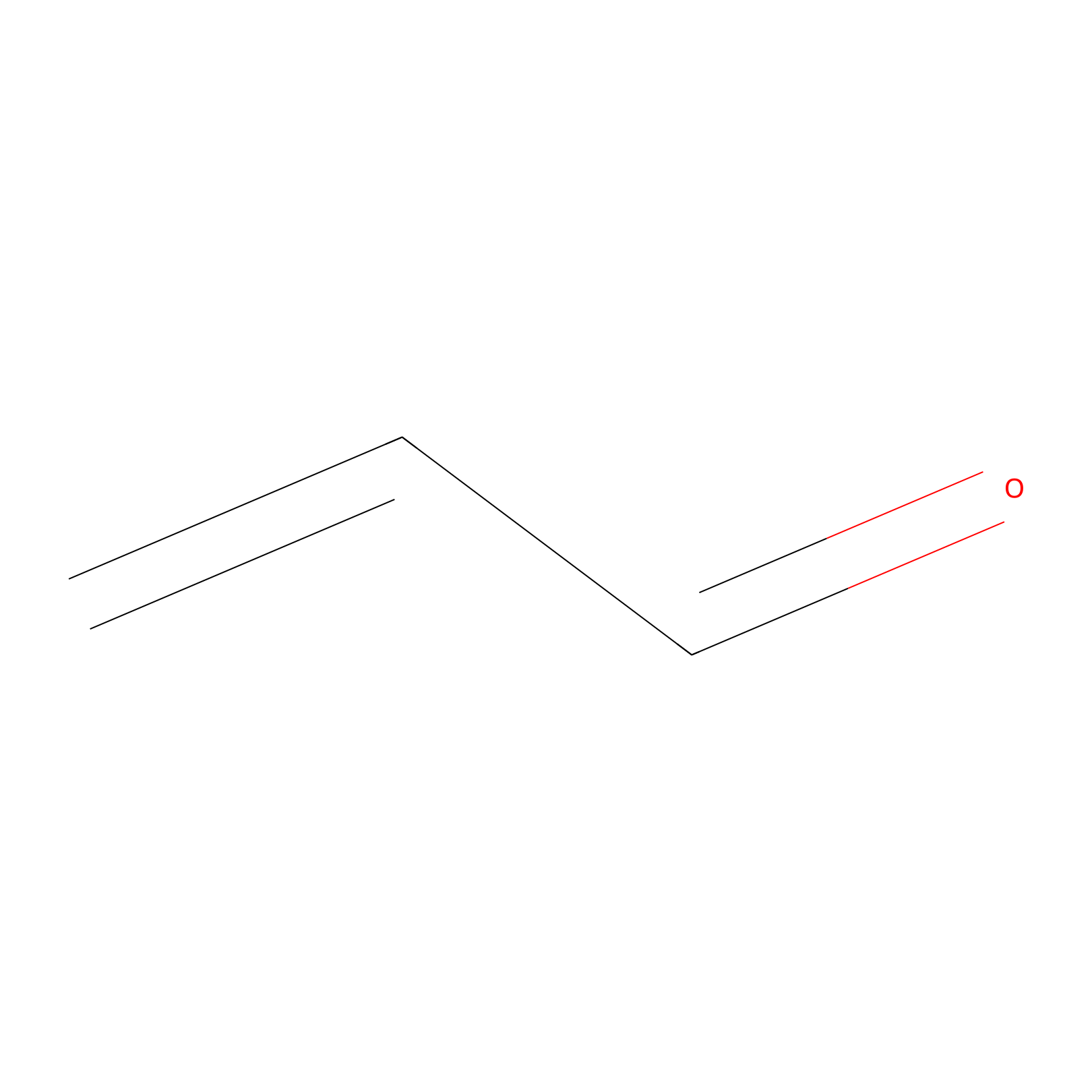 |
N.A. | LDD0223 | [14] | |
|
W1 Probe Info |
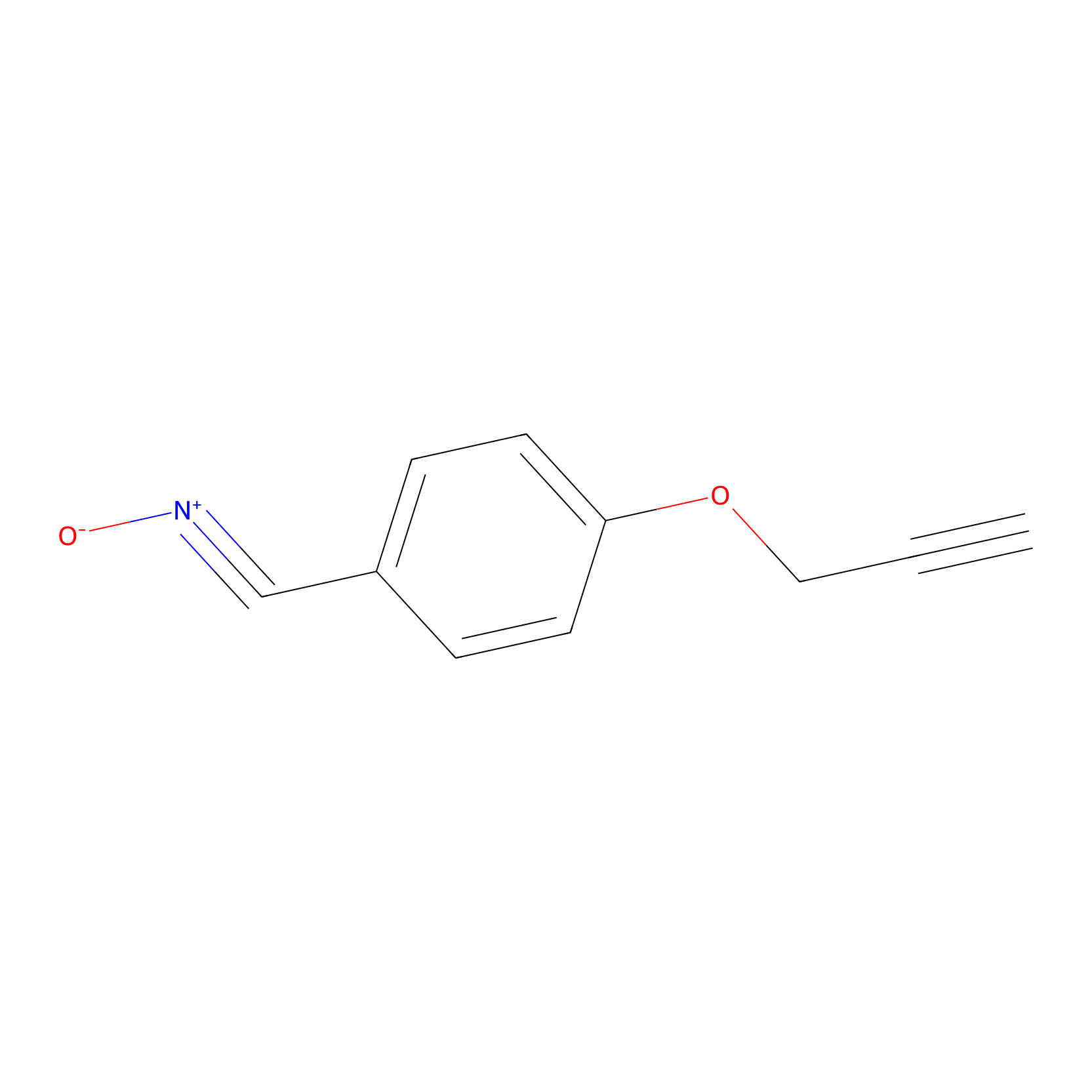 |
K52(0.53); K139(0.59) | LDD0238 | [15] | |
|
5E-2FA Probe Info |
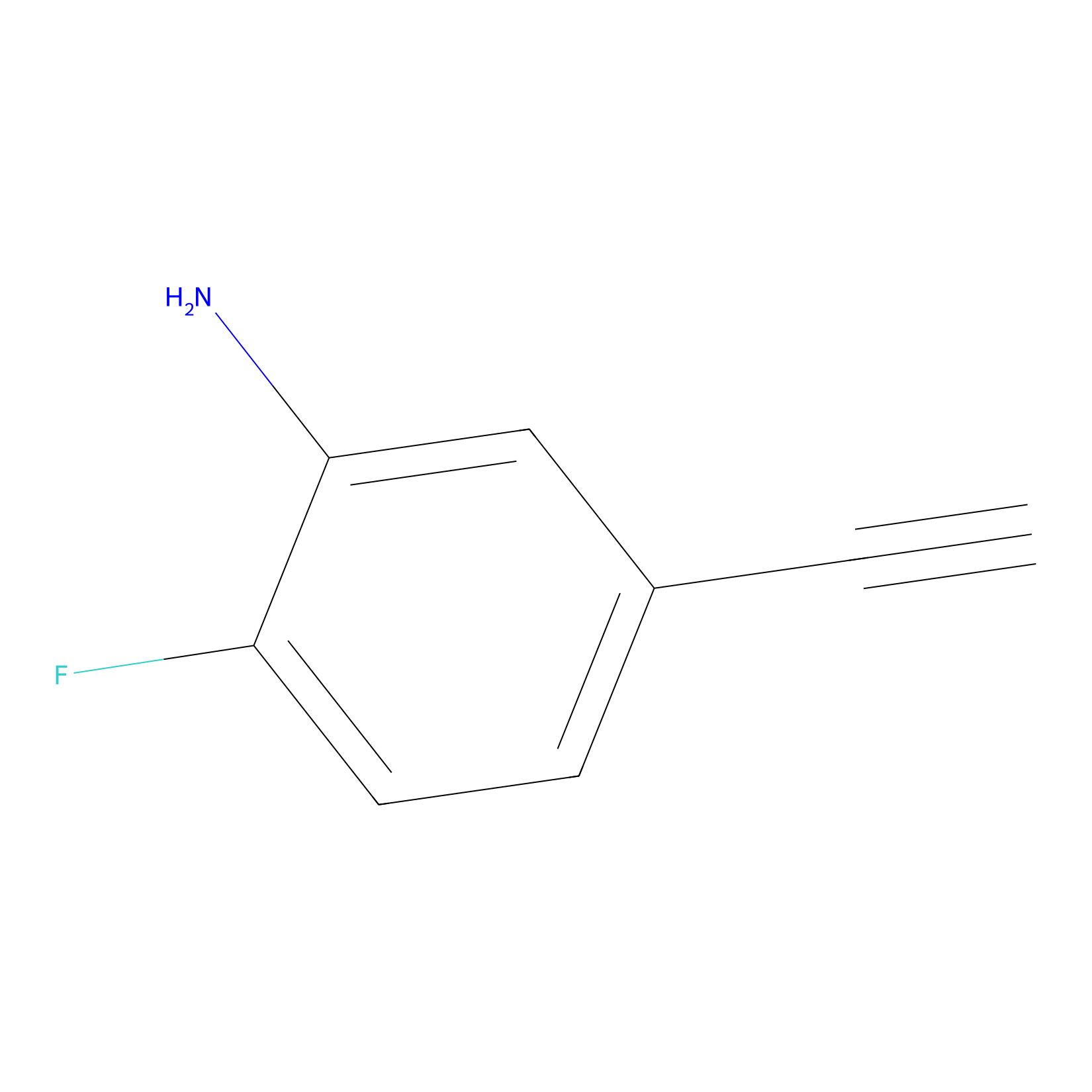 |
N.A. | LDD2235 | [16] | |
|
ATP probe Probe Info |
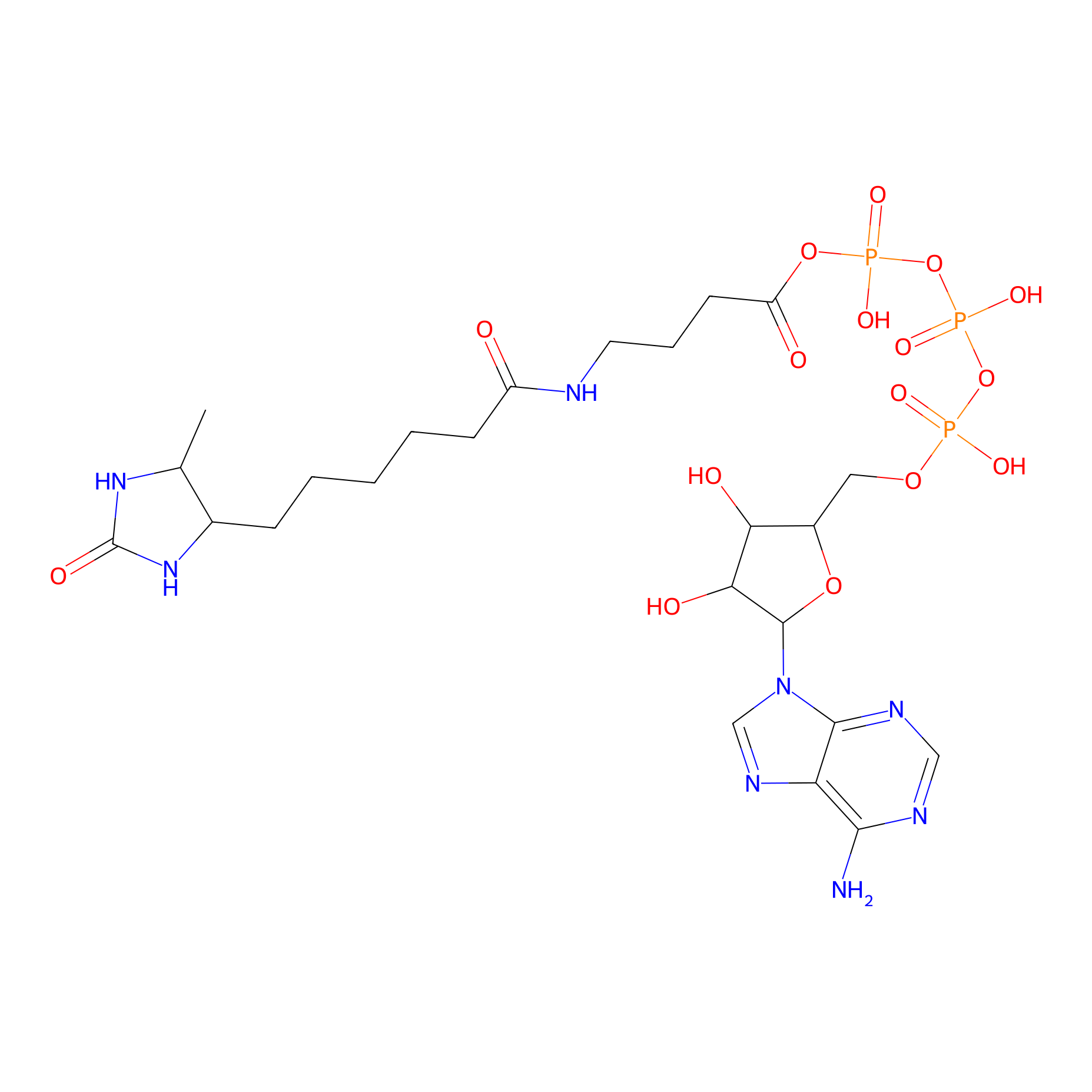 |
K139(0.00); K66(0.00); K30(0.00); K91(0.00) | LDD0199 | [17] | |
|
ATP probe Probe Info |
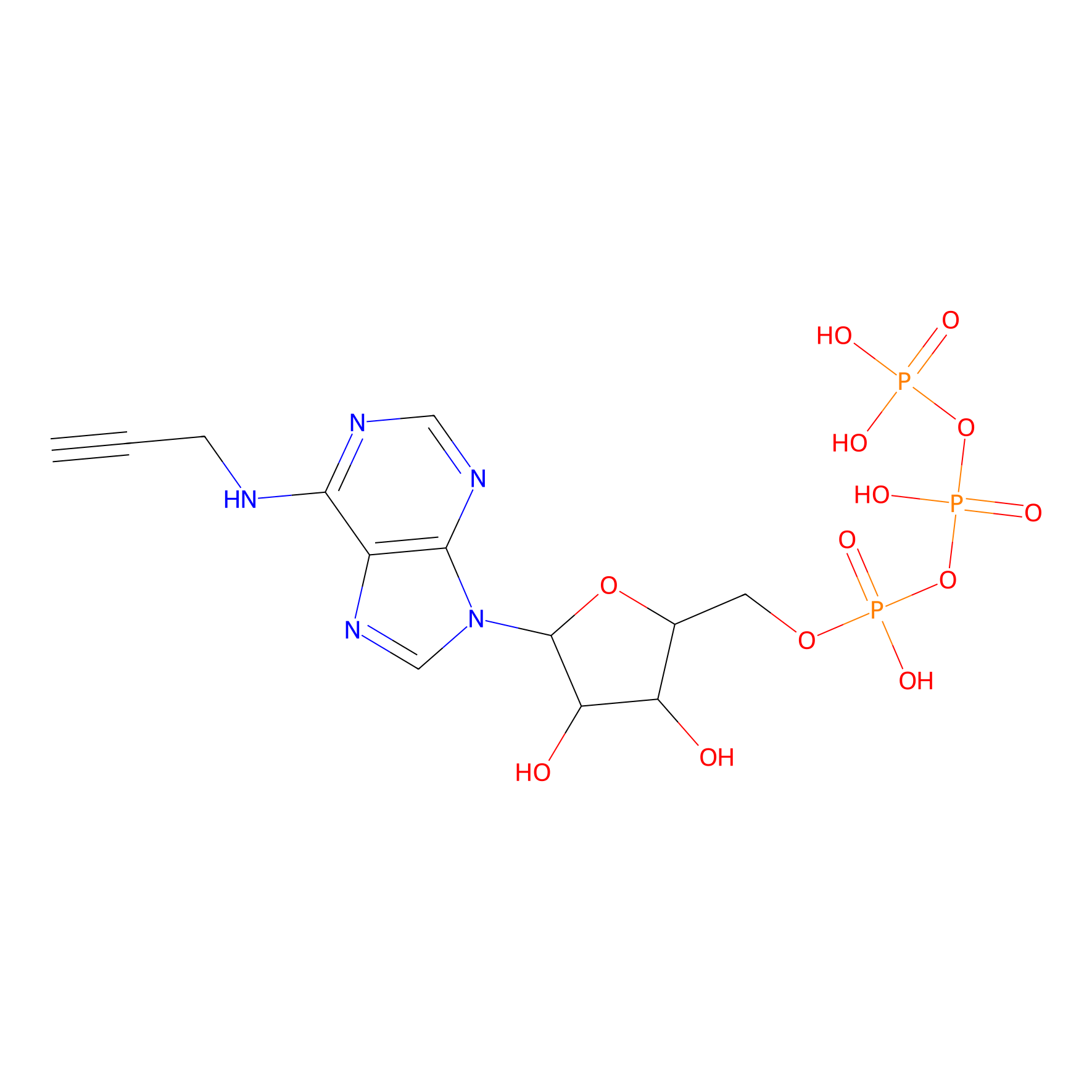 |
K93(0.00); K30(0.00) | LDD0035 | [18] | |
|
NAIA_5 Probe Info |
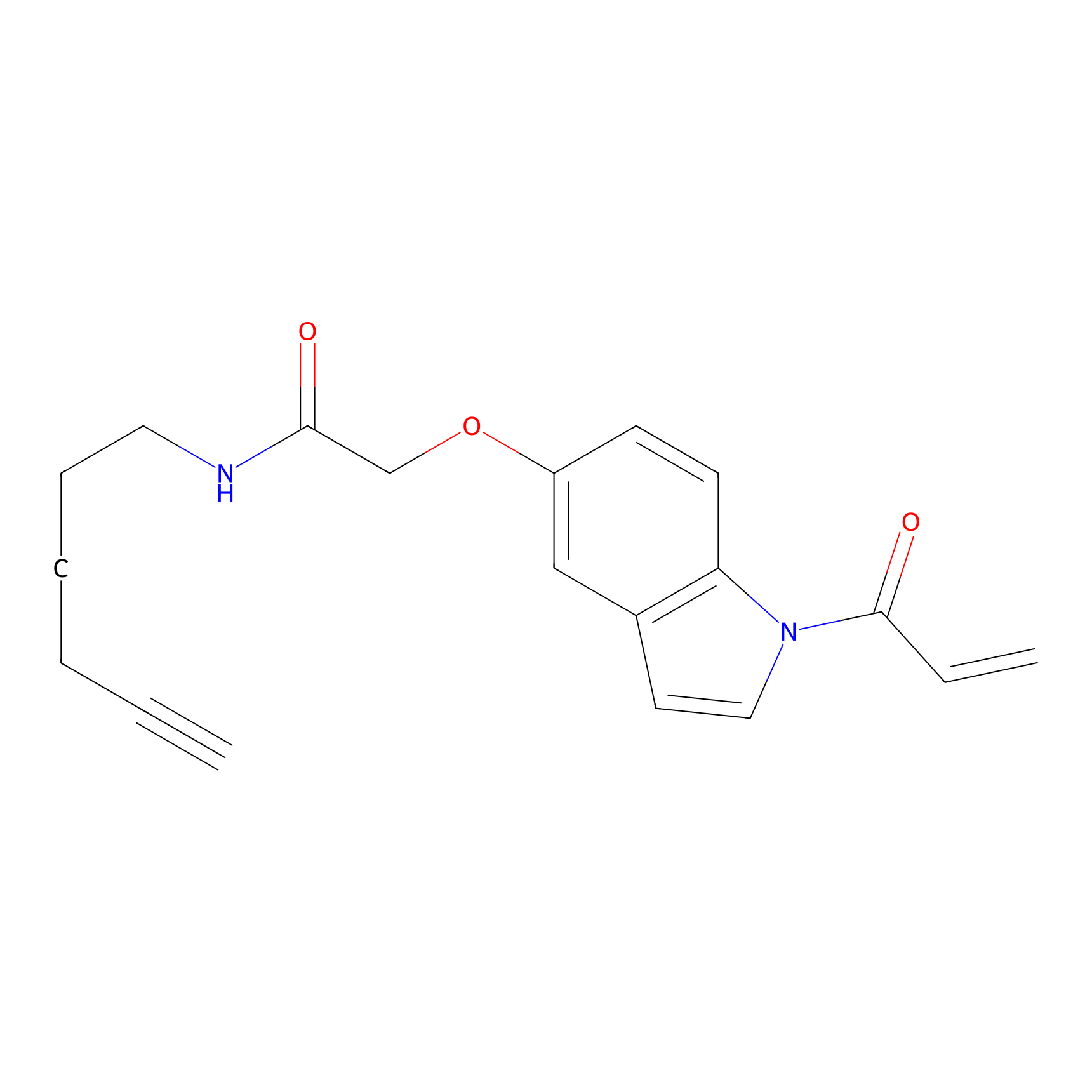 |
N.A. | LDD2224 | [19] | |
|
1d-yne Probe Info |
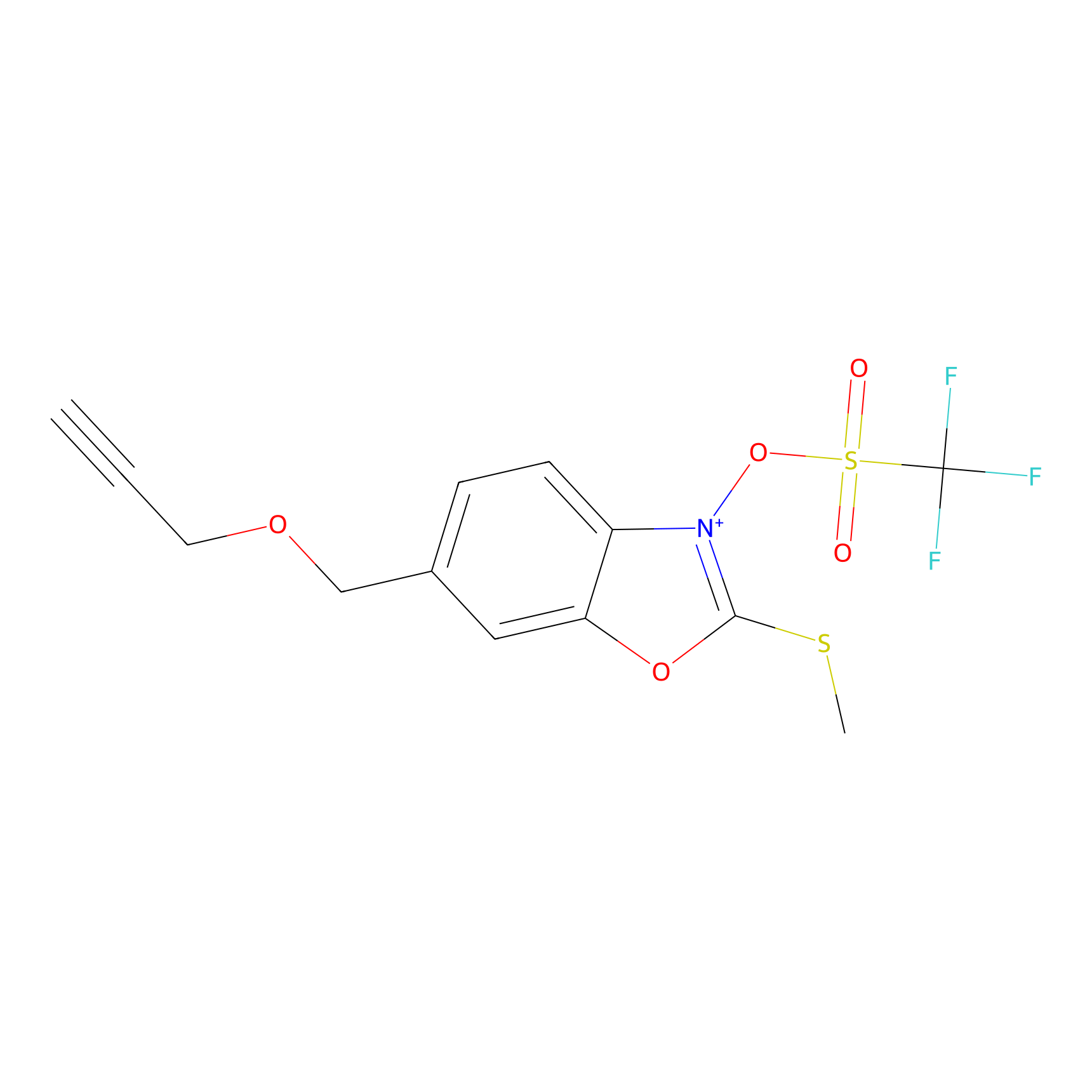 |
K91(0.00); K93(0.00) | LDD0356 | [20] | |
|
NHS Probe Info |
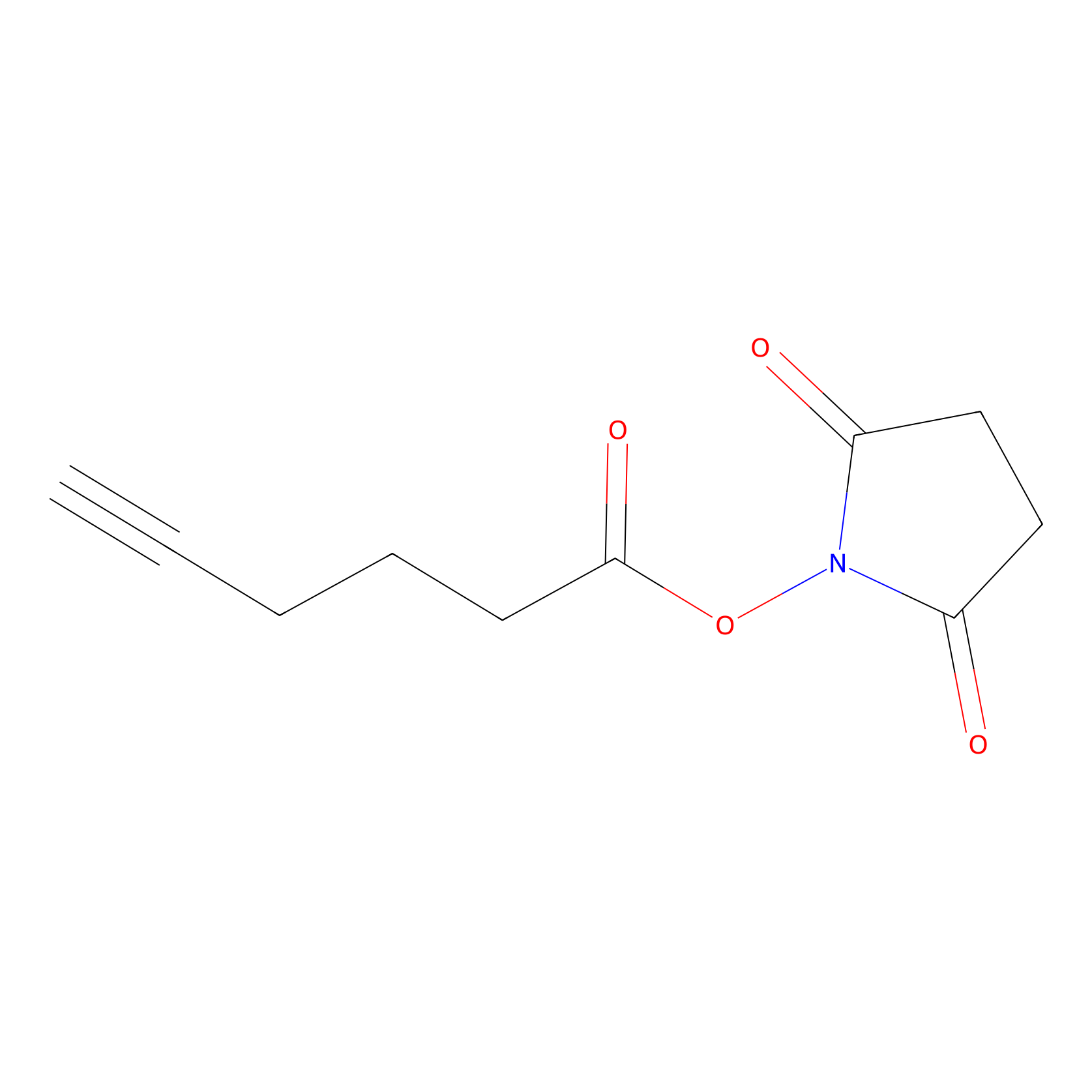 |
K116(0.00); K139(0.00); K66(0.00); K155(0.00) | LDD0010 | [21] | |
|
SF Probe Info |
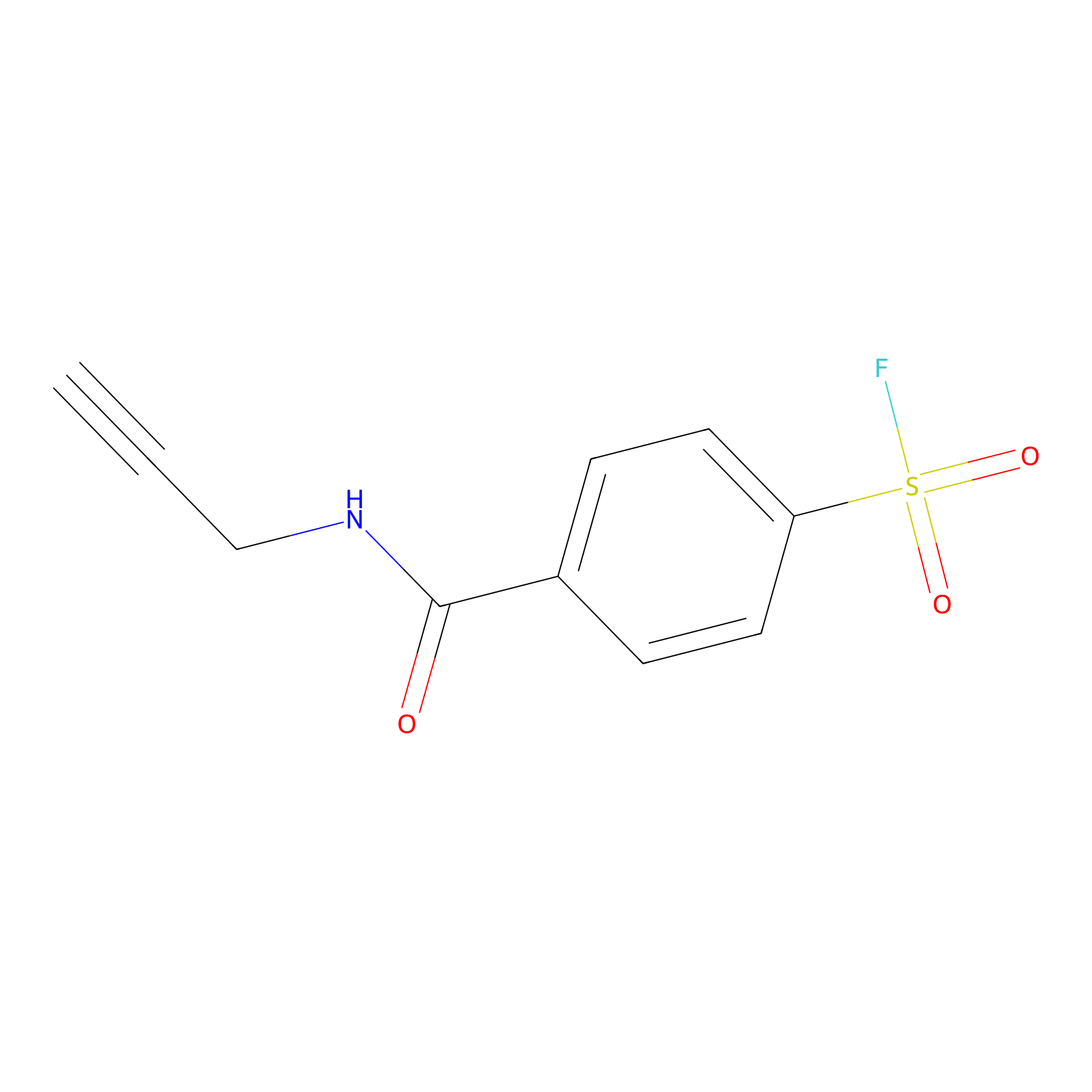 |
K139(0.00); Y35(0.00); K155(0.00); K30(0.00) | LDD0028 | [22] | |
|
STPyne Probe Info |
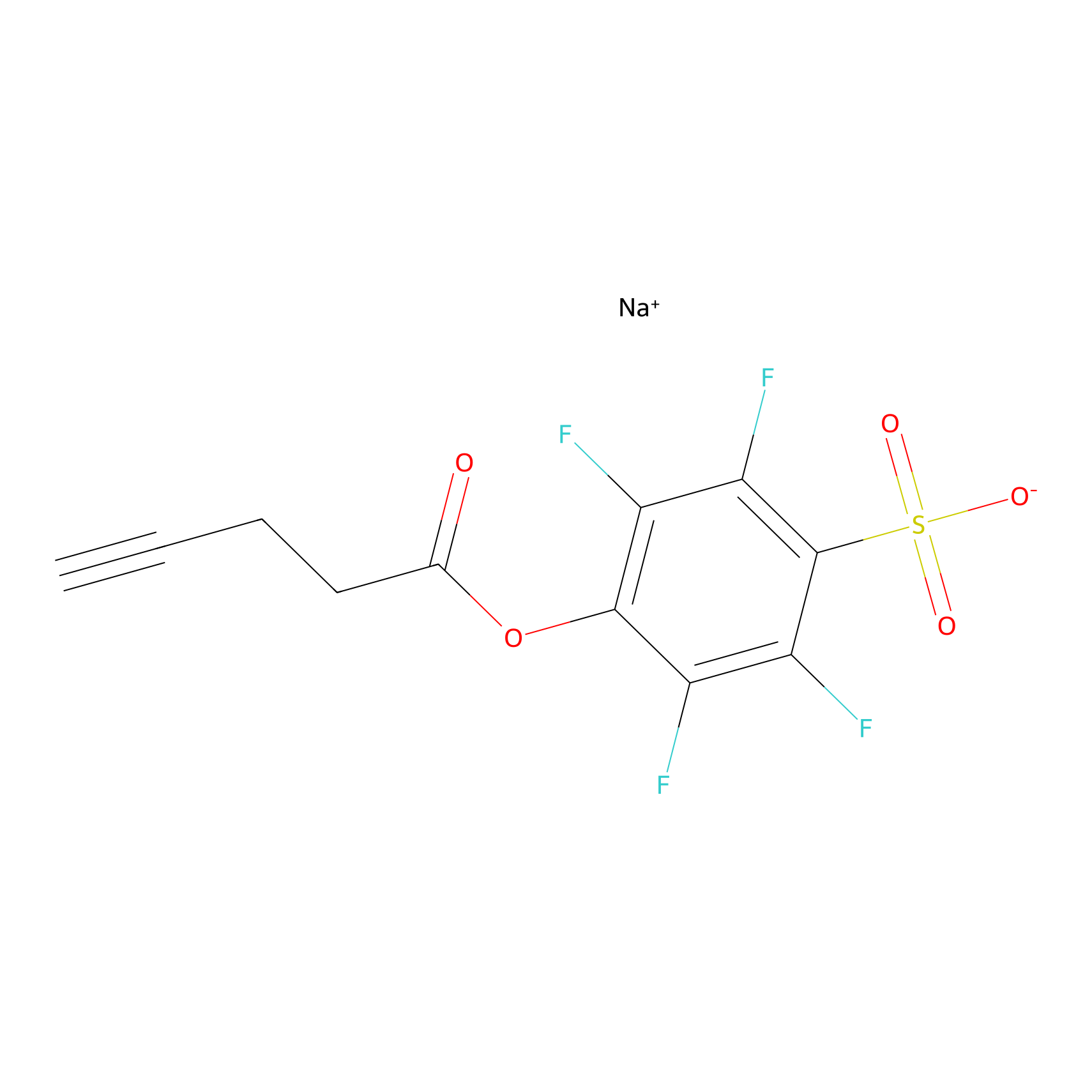 |
K155(0.00); K139(0.00) | LDD0009 | [21] | |
|
Phosphinate-6 Probe Info |
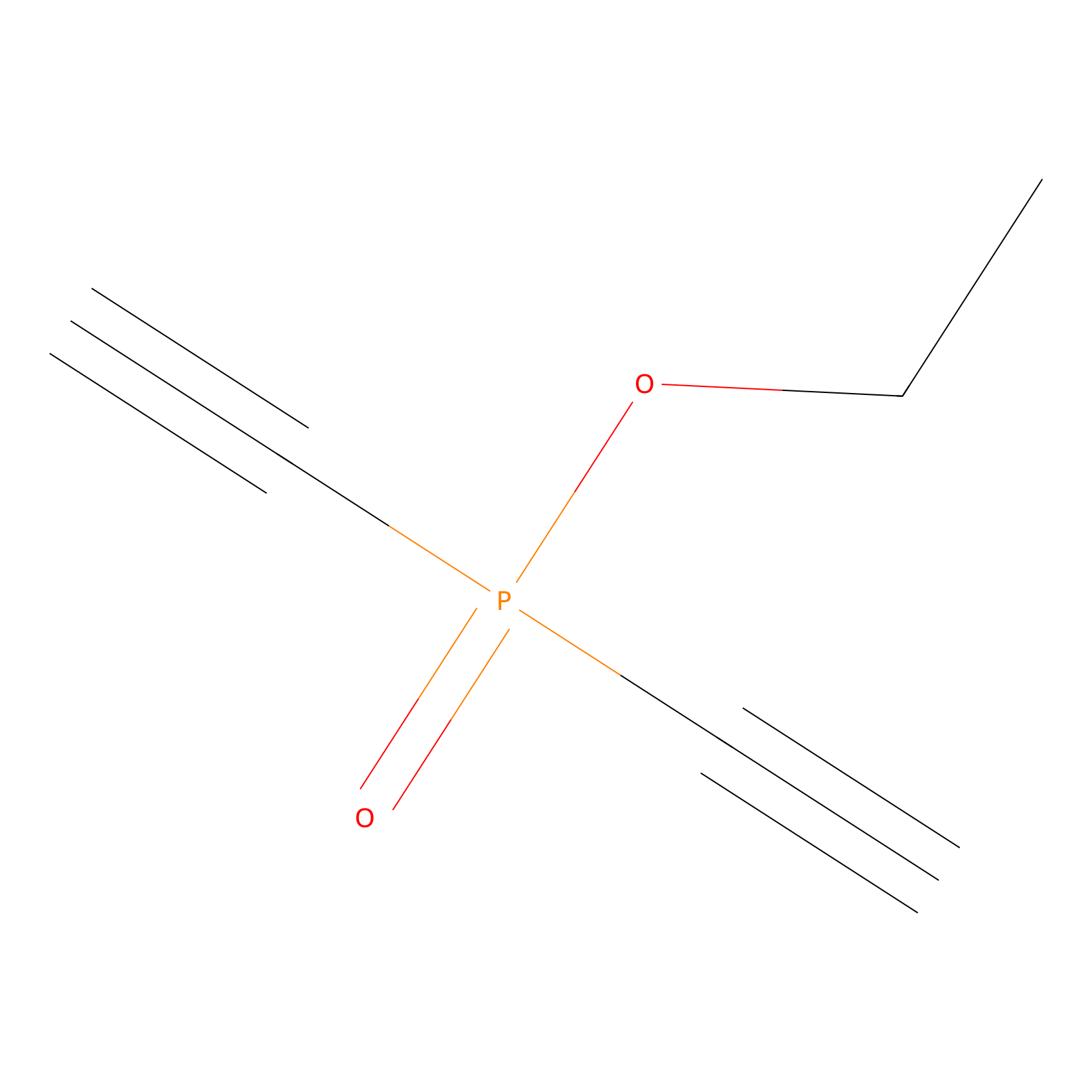 |
N.A. | LDD0018 | [23] | |
|
Ox-W18 Probe Info |
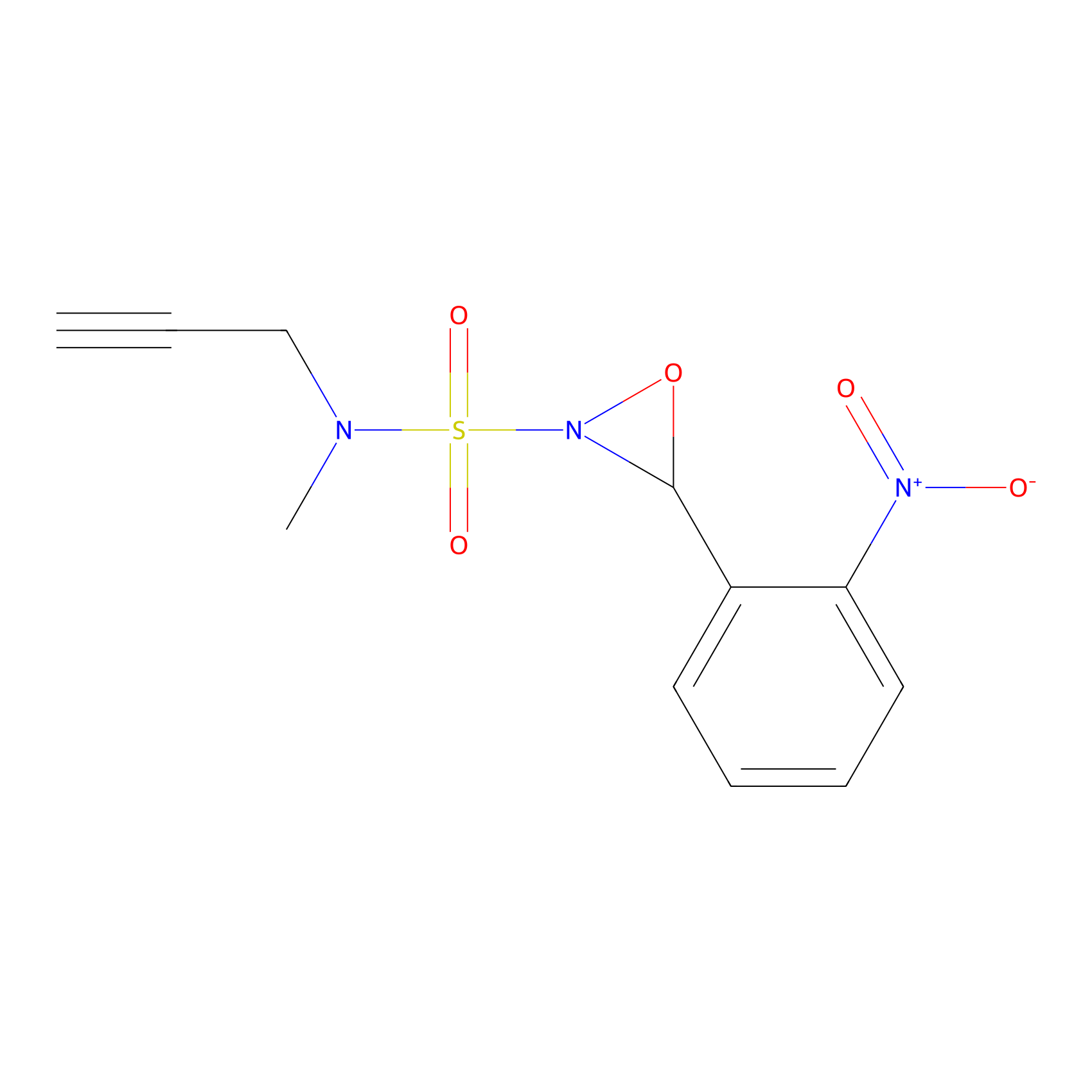 |
N.A. | LDD2175 | [24] | |
|
1c-yne Probe Info |
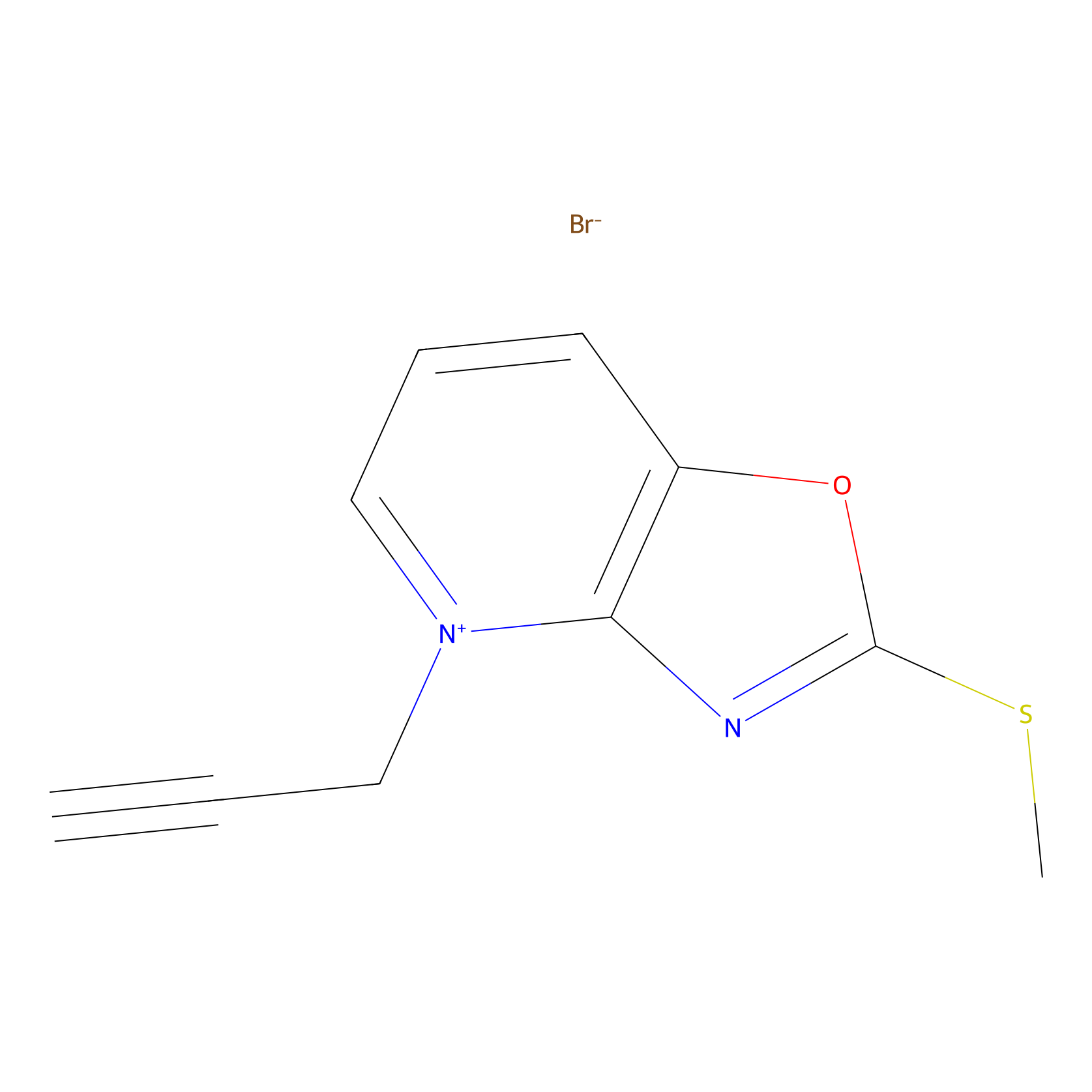 |
K155(0.00); K93(0.00); K30(0.00) | LDD0228 | [20] | |
|
AOyne Probe Info |
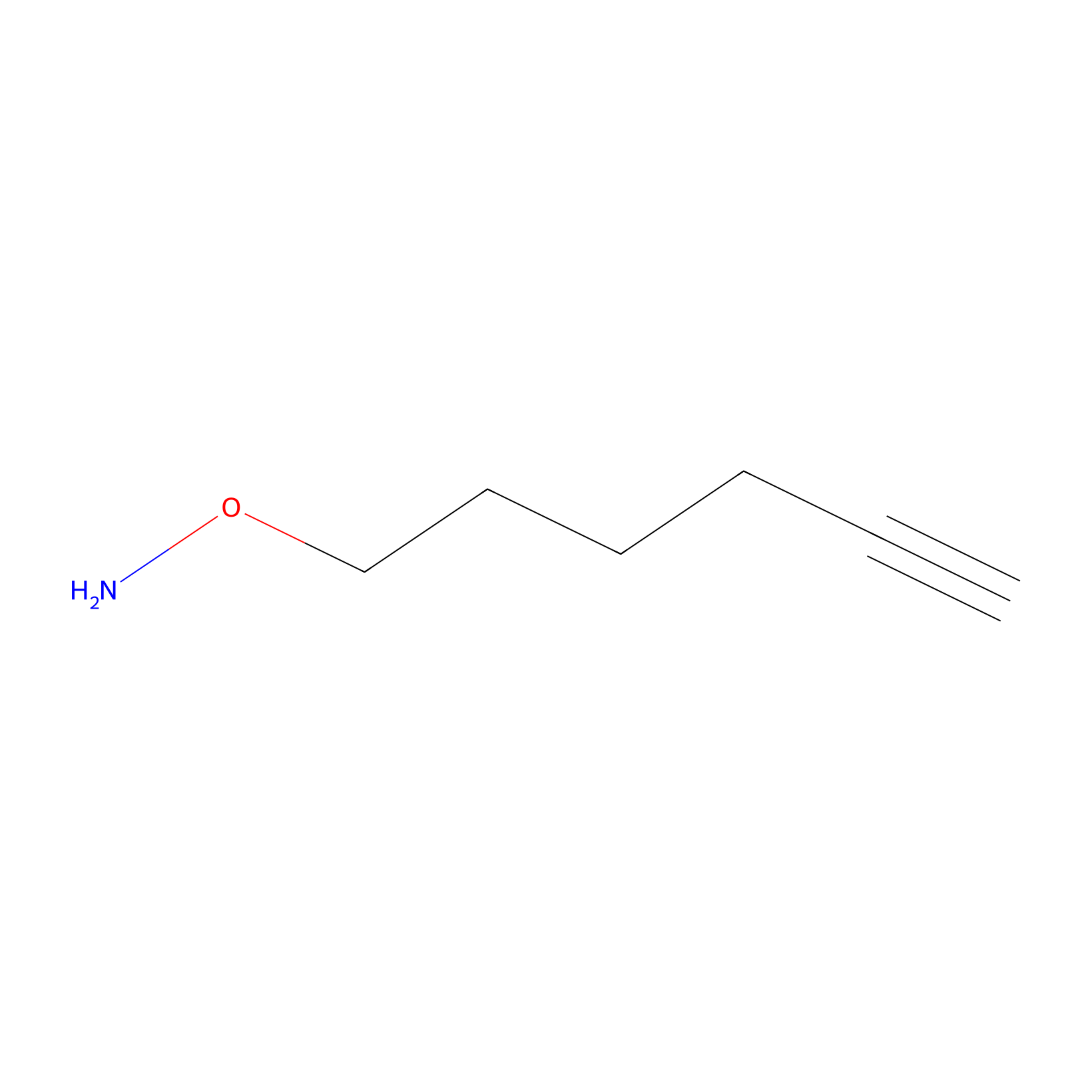 |
12.70 | LDD0443 | [25] | |
|
TER-AC Probe Info |
 |
N.A. | LDD0426 | [26] | |
|
THL-R Probe Info |
 |
N.A. | LDD0077 | [27] | |
|
HHS-482 Probe Info |
 |
Y13(1.05); Y96(1.11) | LDD2239 | [13] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
STS-2 Probe Info |
 |
N.A. | LDD0138 | [28] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0156 | Aniline | NCI-H1299 | 11.72 | LDD0403 | [1] |
| LDCM0151 | AZ-11 | HeLa | 10.00 | LDD2154 | [11] |
| LDCM0116 | HHS-0101 | DM93 | Y96(0.65) | LDD0264 | [12] |
| LDCM0117 | HHS-0201 | DM93 | Y96(0.75) | LDD0265 | [12] |
| LDCM0118 | HHS-0301 | DM93 | Y96(1.09) | LDD0266 | [12] |
| LDCM0119 | HHS-0401 | DM93 | Y96(1.18) | LDD0267 | [12] |
| LDCM0120 | HHS-0701 | DM93 | Y96(0.88) | LDD0268 | [12] |
| LDCM0022 | KB02 | HEC-1 | C169(4.76) | LDD2350 | [10] |
| LDCM0023 | KB03 | HEC-1 | C169(6.97) | LDD2767 | [10] |
| LDCM0024 | KB05 | SKN | C169(1.61) | LDD3432 | [10] |
| LDCM0109 | NEM | HeLa | N.A. | LDD0223 | [14] |
| LDCM0018 | Orlistat | Hep-G2 | N.A. | LDD0077 | [27] |
| LDCM0111 | W14 | Hep-G2 | K52(0.53); K139(0.59) | LDD0238 | [15] |
| LDCM0112 | W16 | Hep-G2 | R54(0.95); Q140(1.07) | LDD0239 | [15] |
| LDCM0113 | W17 | Hep-G2 | K139(0.50); Q140(0.50); R54(0.53) | LDD0240 | [15] |
The Interaction Atlas With This Target
References
