Details of the Target
General Information of Target
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
TH211 Probe Info |
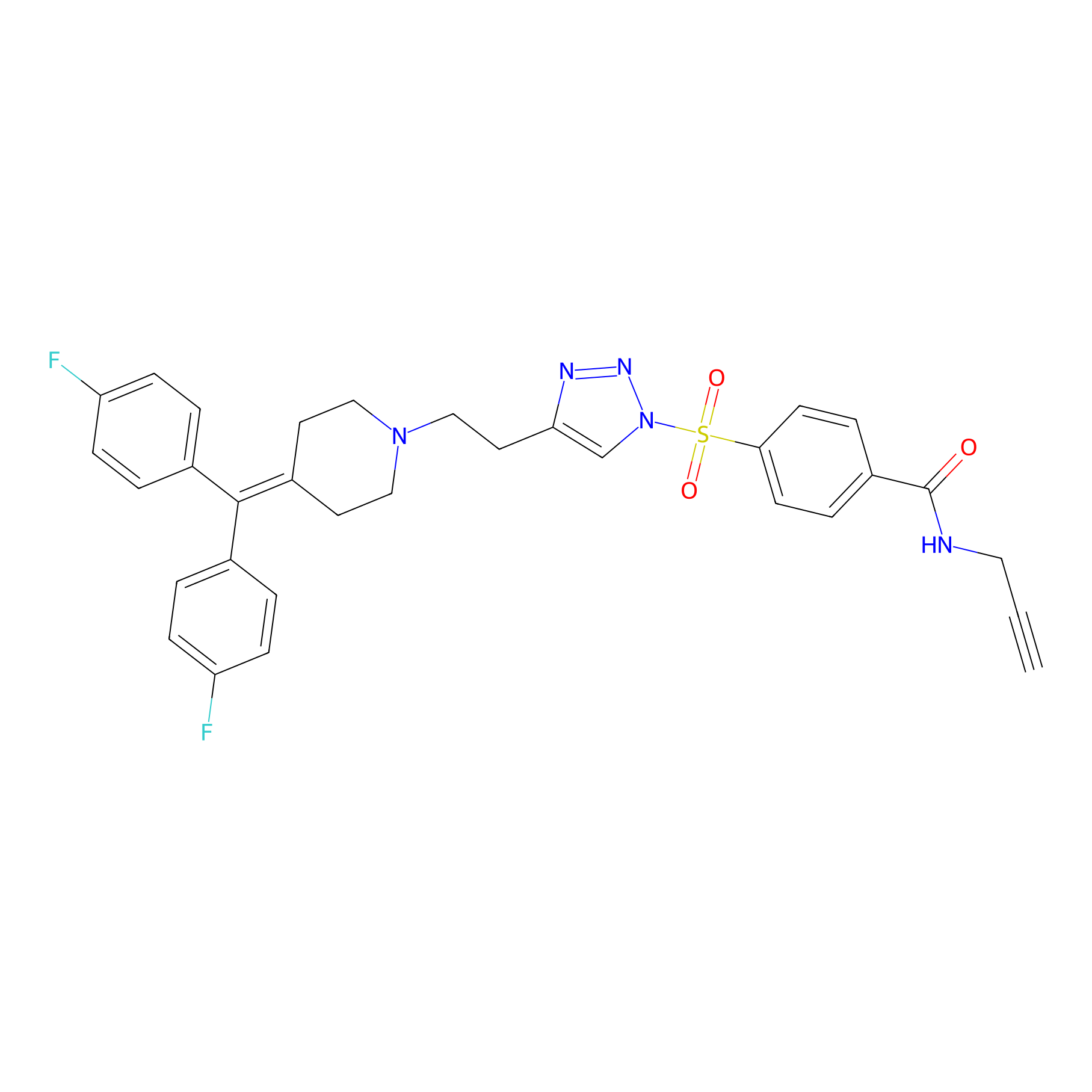 |
Y515(20.00); Y518(20.00) | LDD0260 | [1] | |
|
BTD Probe Info |
 |
C586(1.23); C488(0.93) | LDD2099 | [2] | |
|
DBIA Probe Info |
 |
C572(6.40) | LDD0209 | [3] | |
|
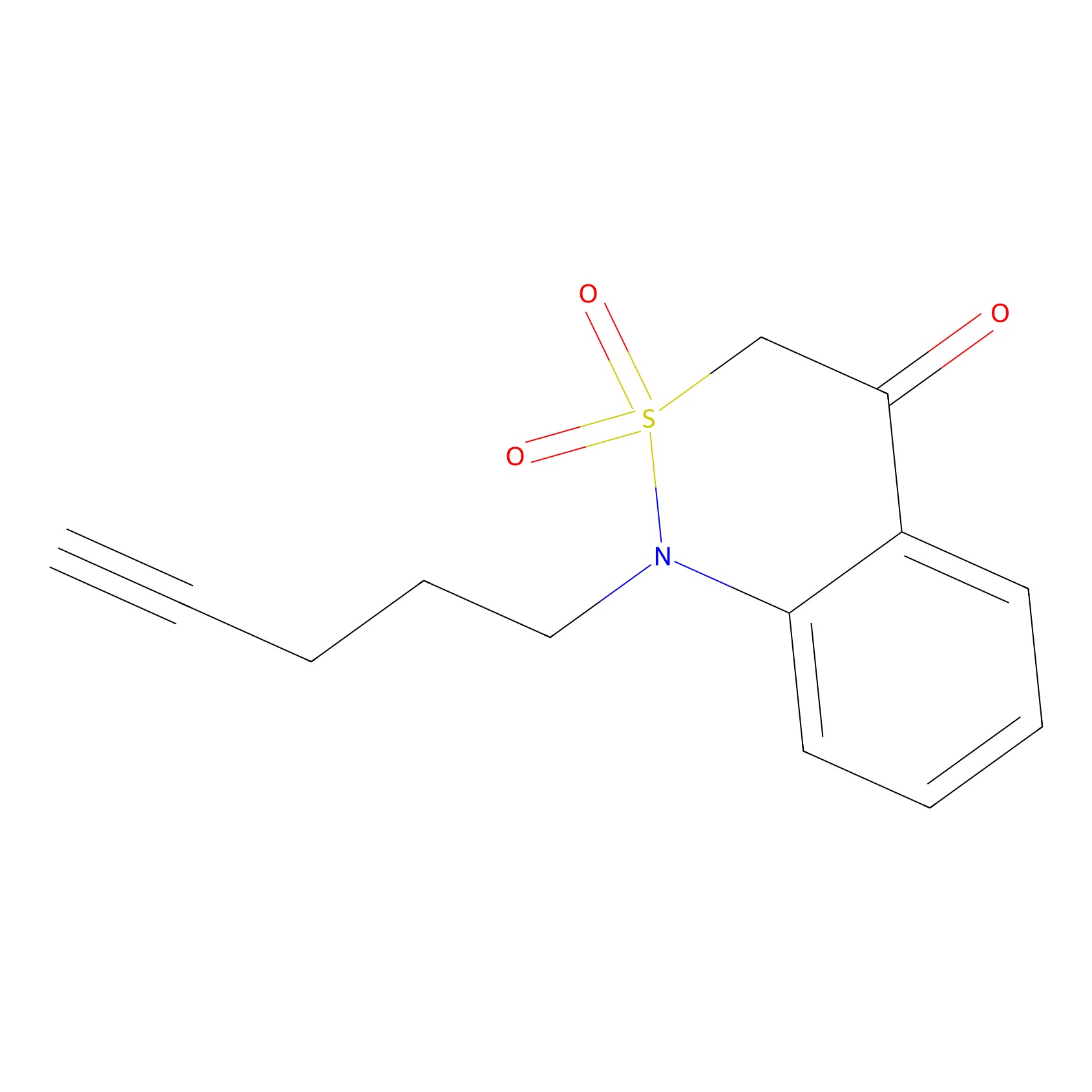
IPM Probe Info |
 |
C586(4.05) | LDD1701 | [2] | |
|
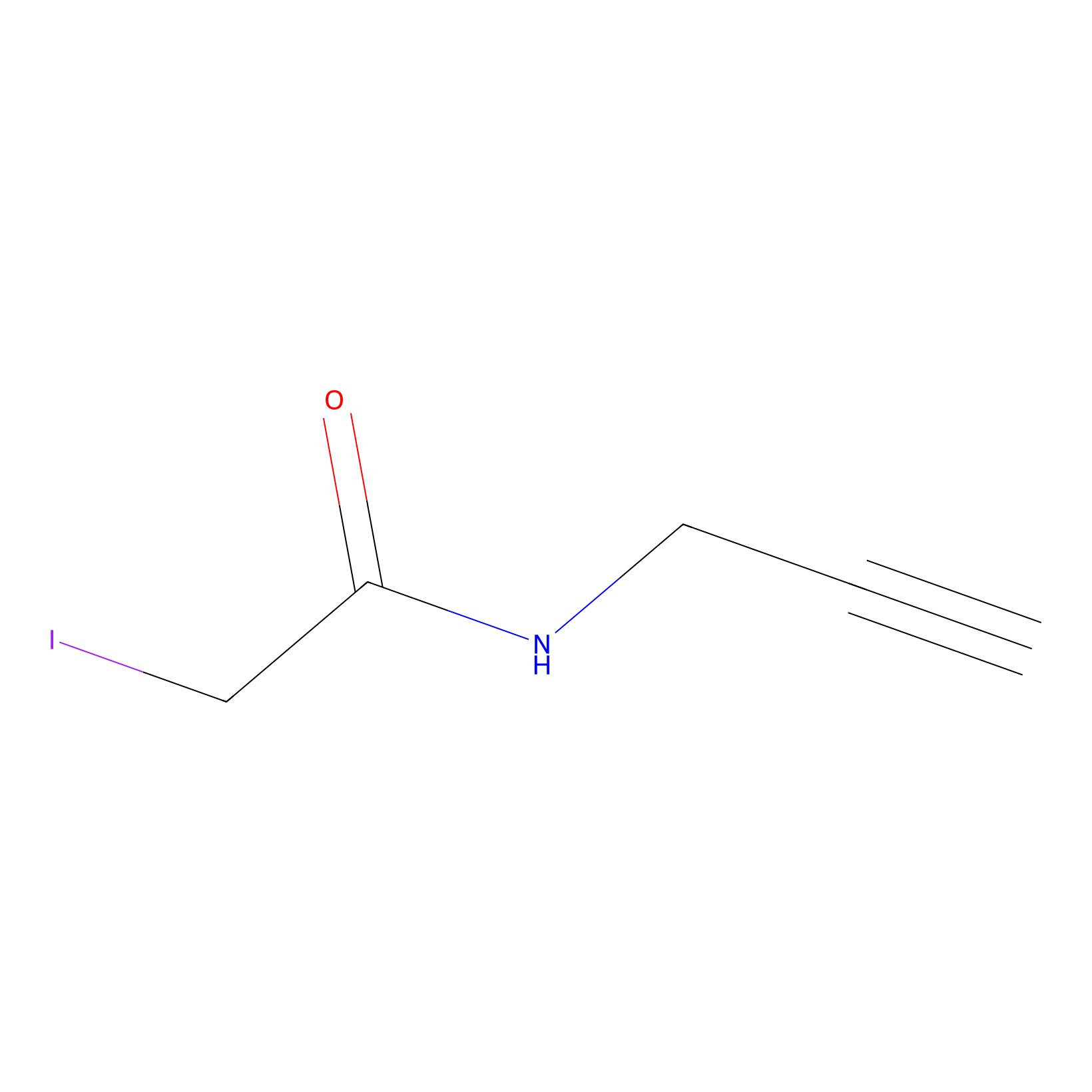
4-Iodoacetamidophenylacetylene Probe Info |
 |
C217(0.00); C386(0.00); C488(0.00); C586(0.00) | LDD0038 | [4] | |
|
IA-alkyne Probe Info |
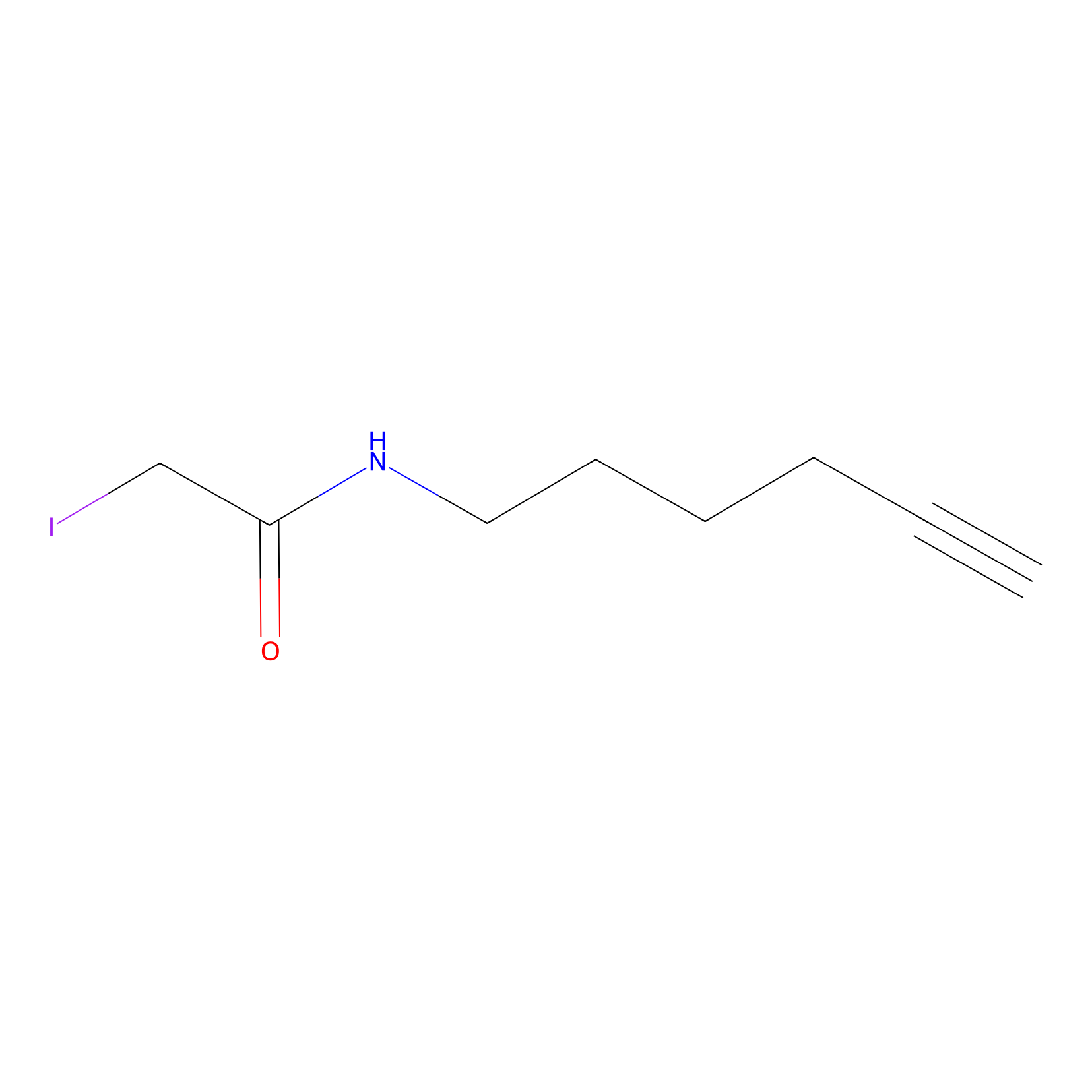 |
C217(0.00); C572(0.00); C409(0.00); C132(0.00) | LDD0036 | [4] | |
|
IPIAA_L Probe Info |
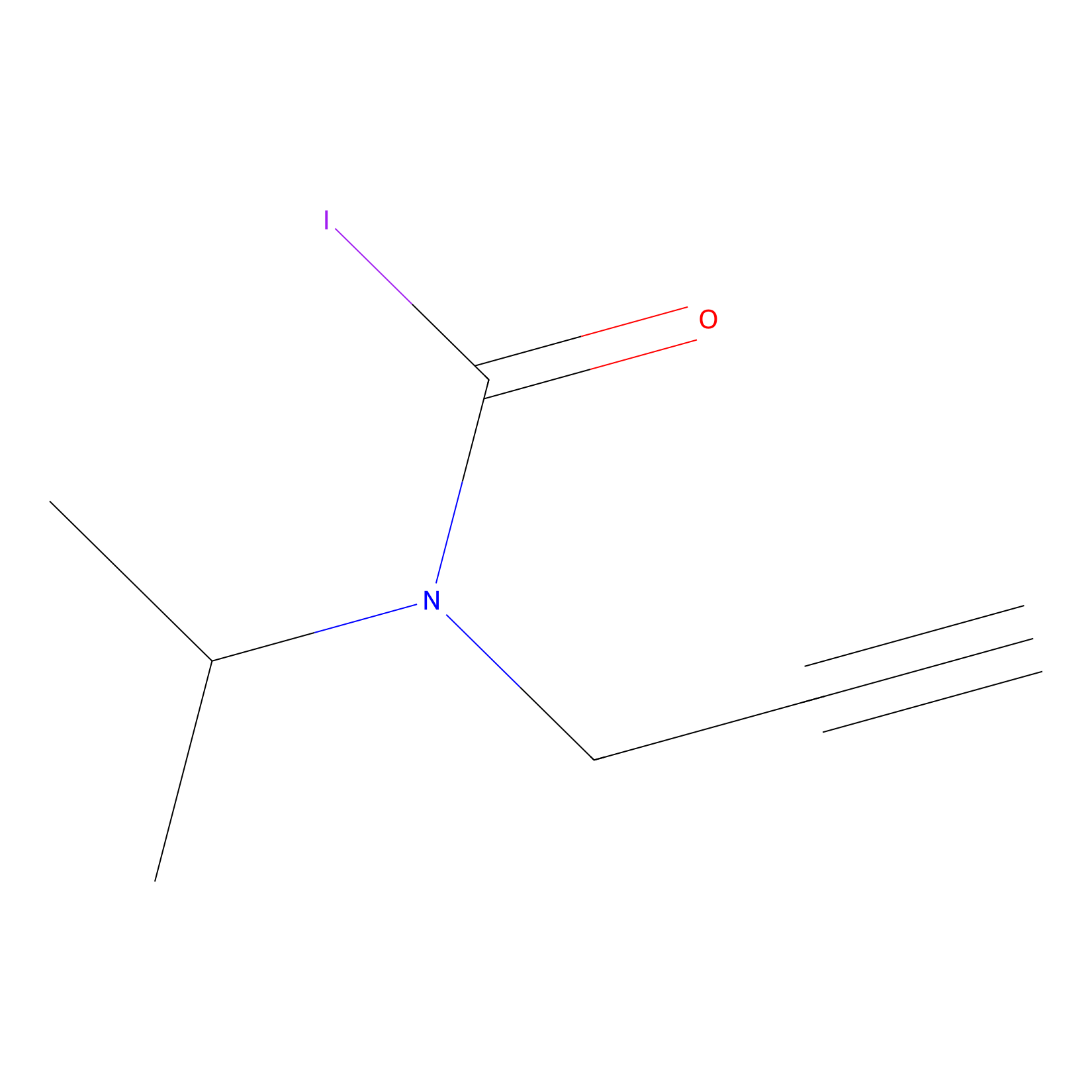 |
N.A. | LDD0031 | [5] | |
|
Lodoacetamide azide Probe Info |
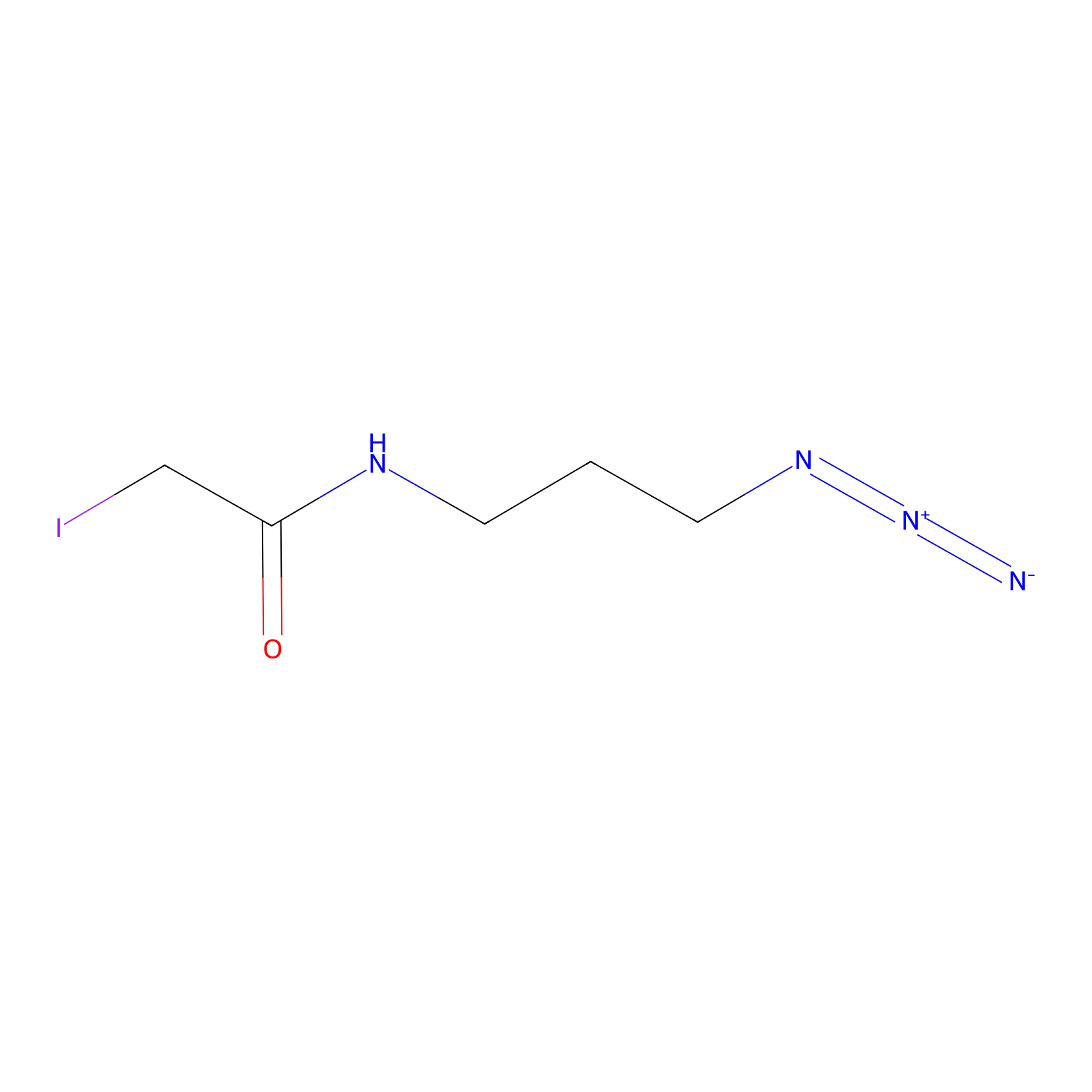 |
C217(0.00); C386(0.00); C572(0.00); C488(0.00) | LDD0037 | [4] | |
|
WYneN Probe Info |
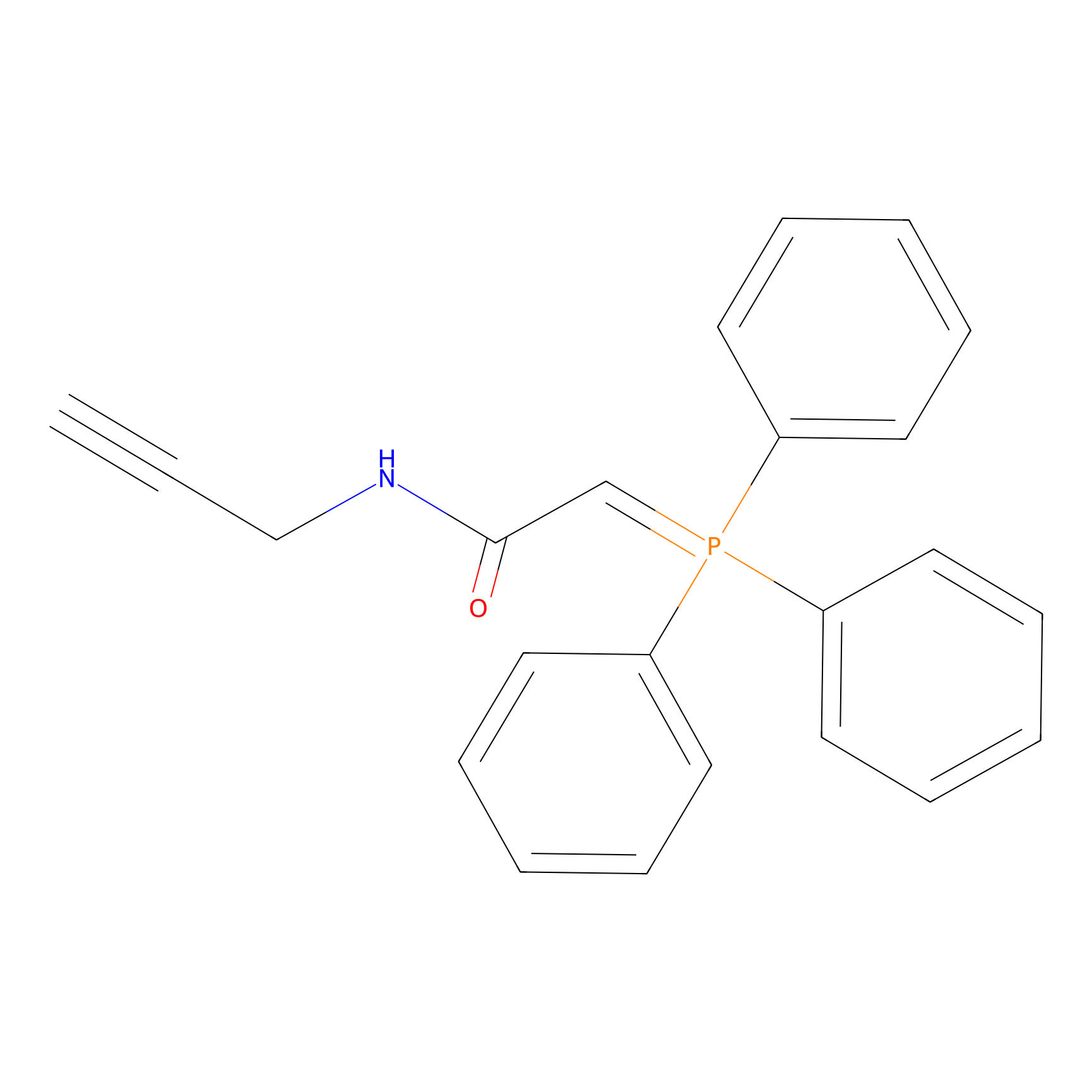 |
N.A. | LDD0021 | [6] | |
|
WYneO Probe Info |
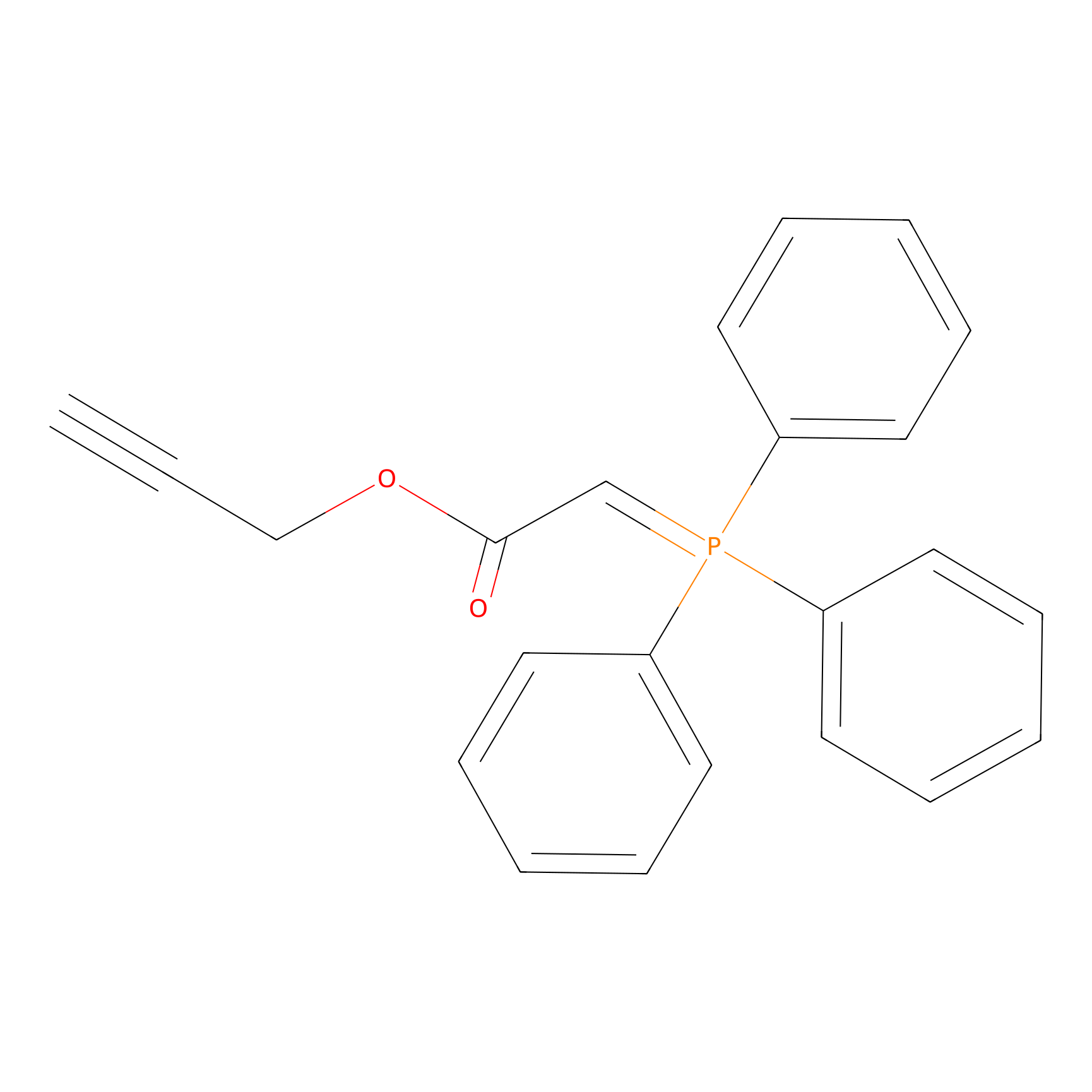 |
N.A. | LDD0022 | [6] | |
|
TFBX Probe Info |
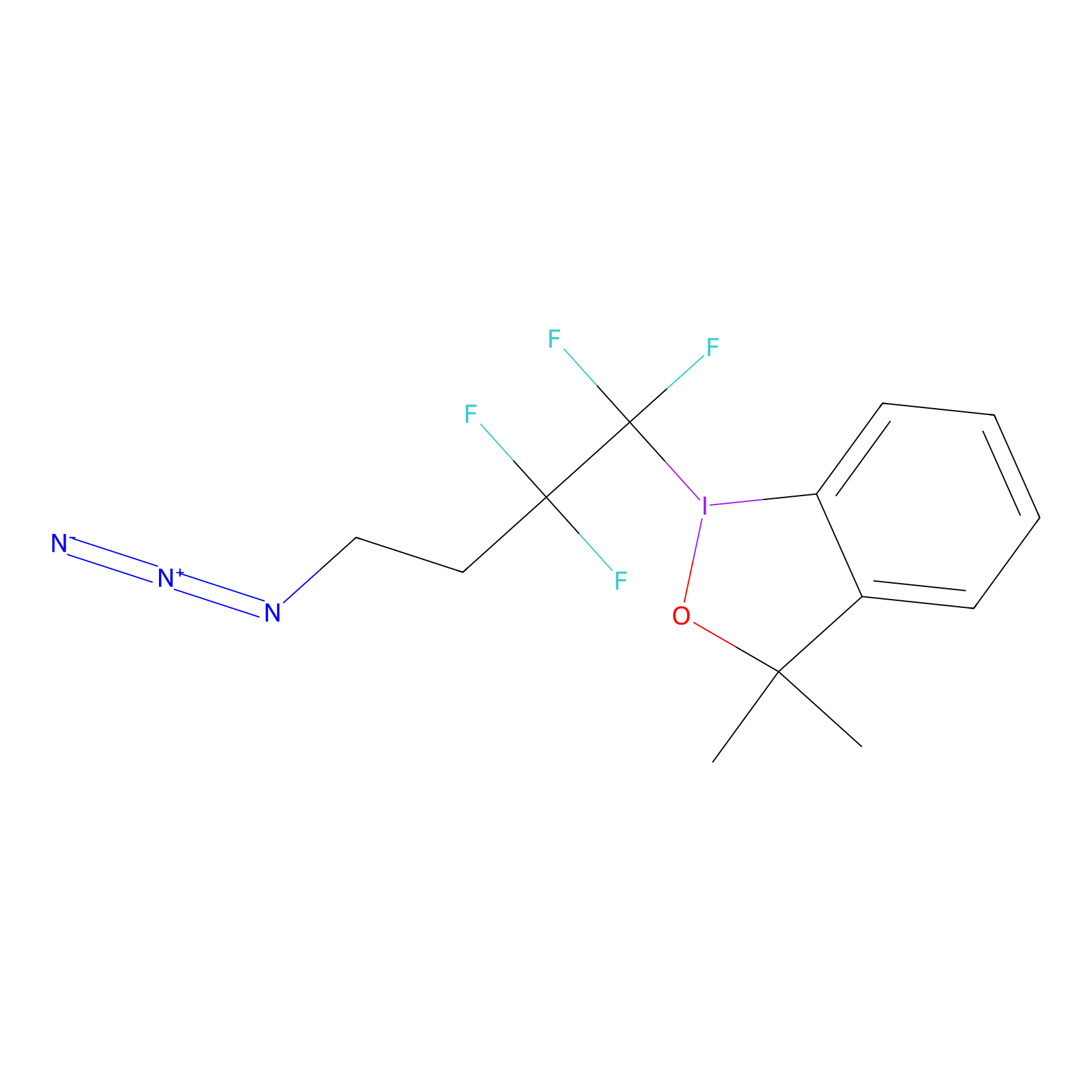 |
C488(0.00); C502(0.00) | LDD0148 | [7] | |
|
W1 Probe Info |
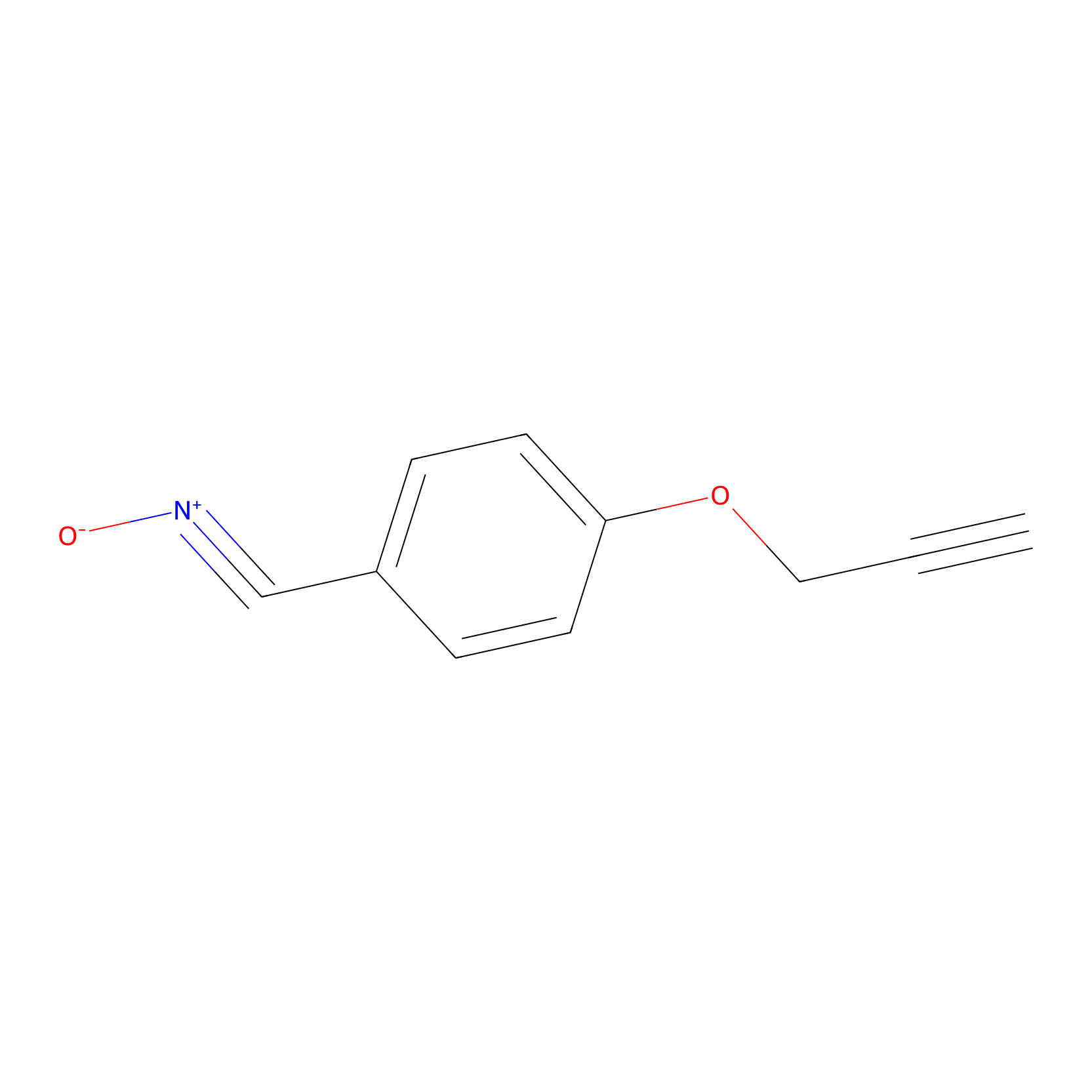 |
N.A. | LDD0236 | [8] | |
|
NAIA_5 Probe Info |
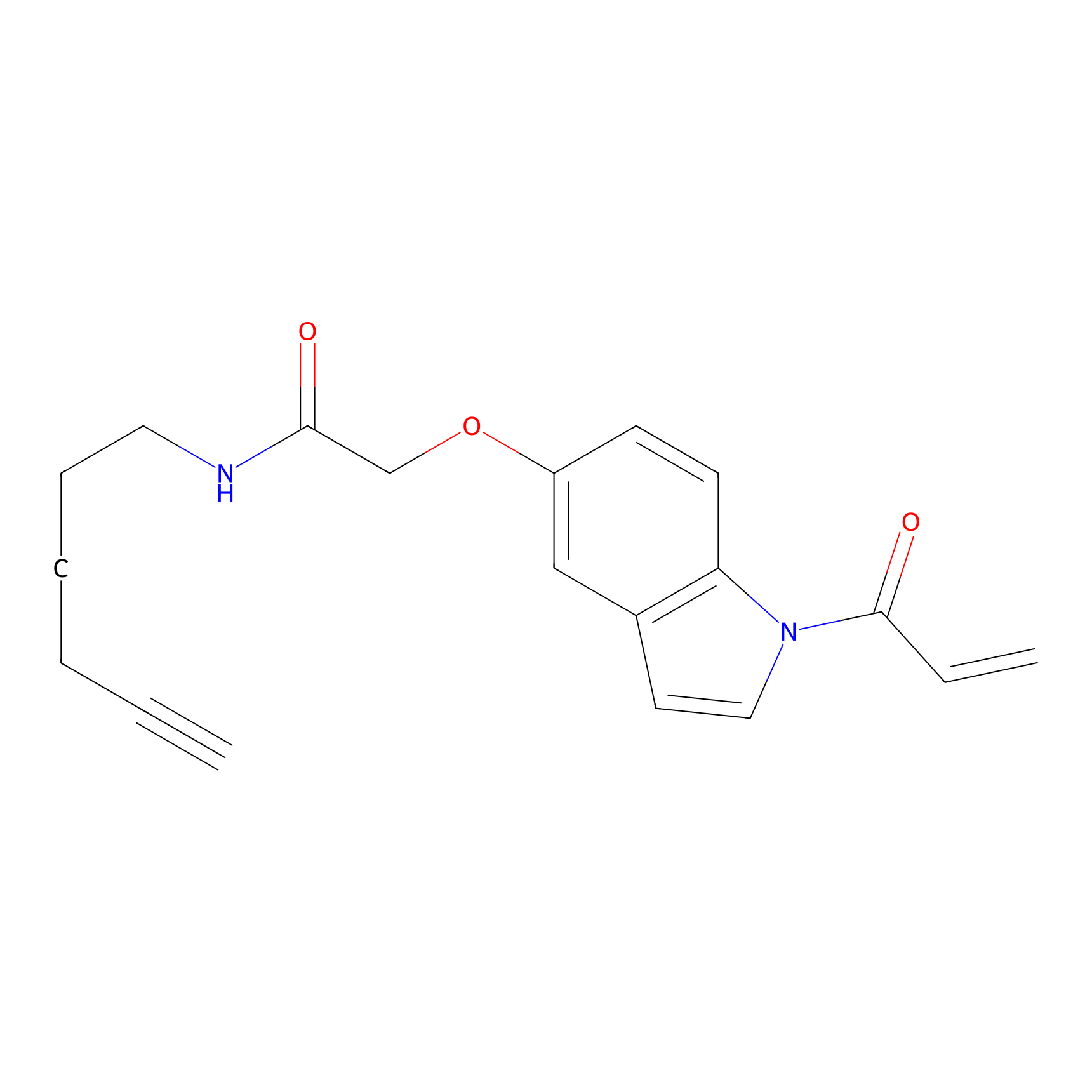 |
N.A. | LDD2223 | [9] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
C218 Probe Info |
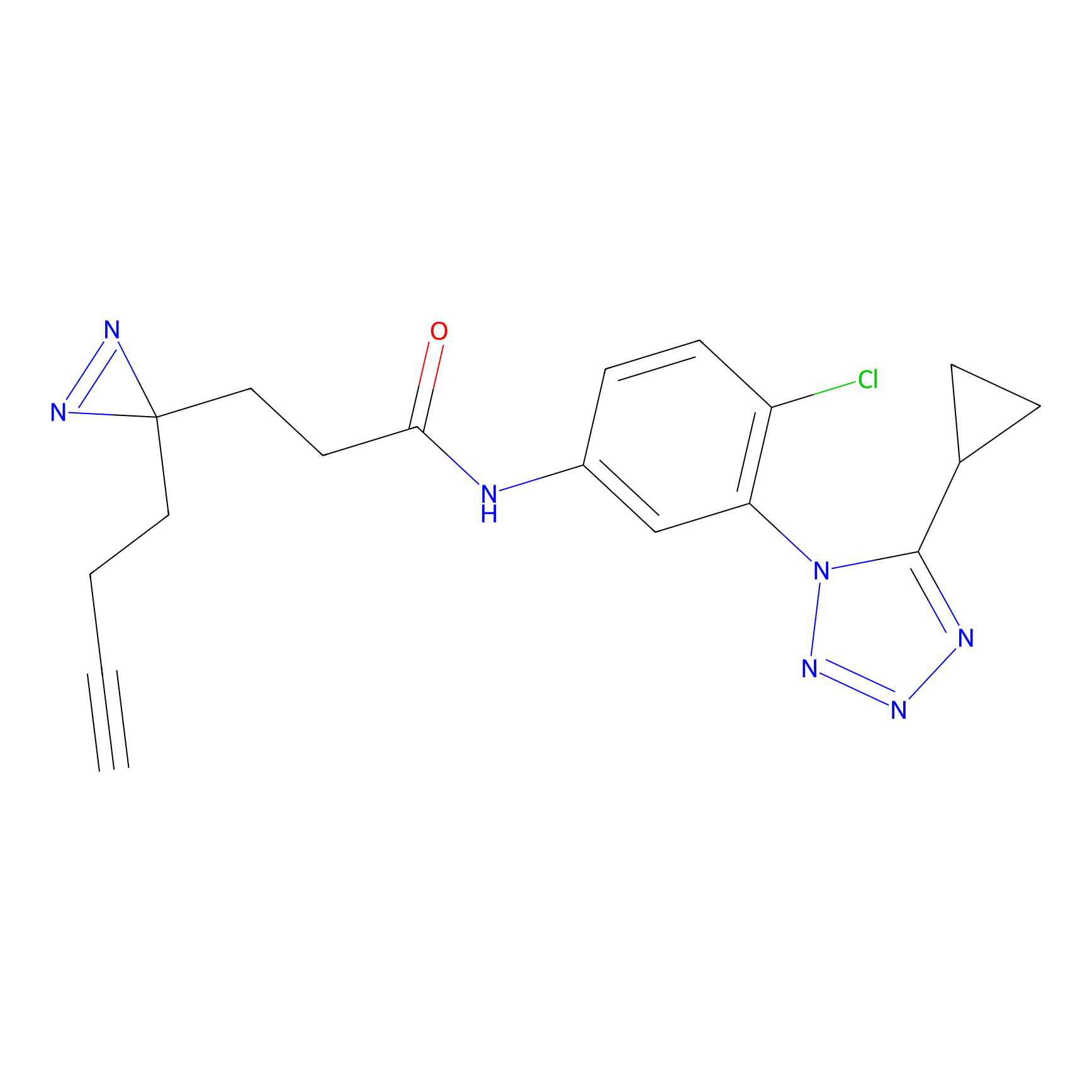 |
13.83 | LDD1892 | [10] | |
|
C361 Probe Info |
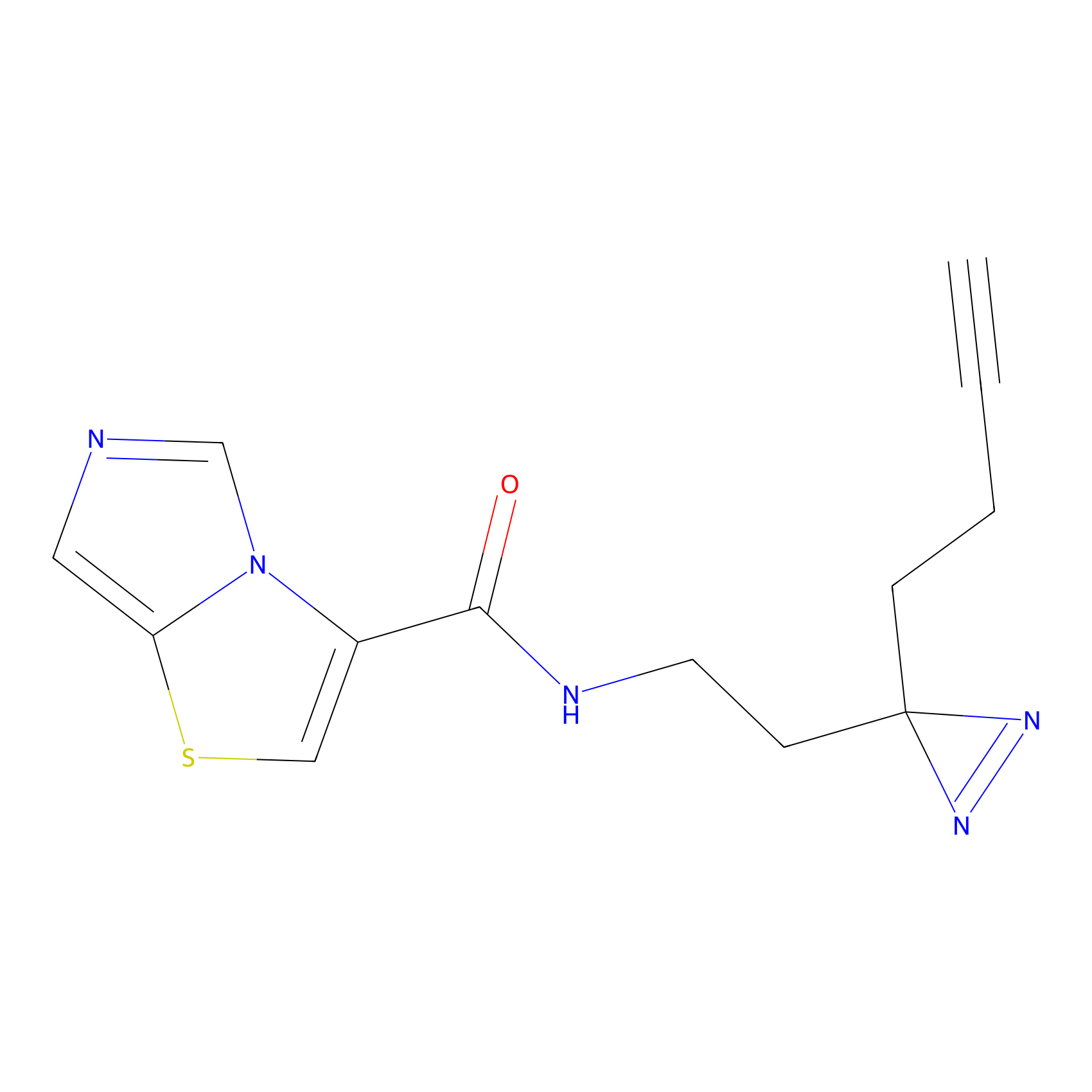 |
19.16 | LDD2022 | [10] | |
|
FFF probe3 Probe Info |
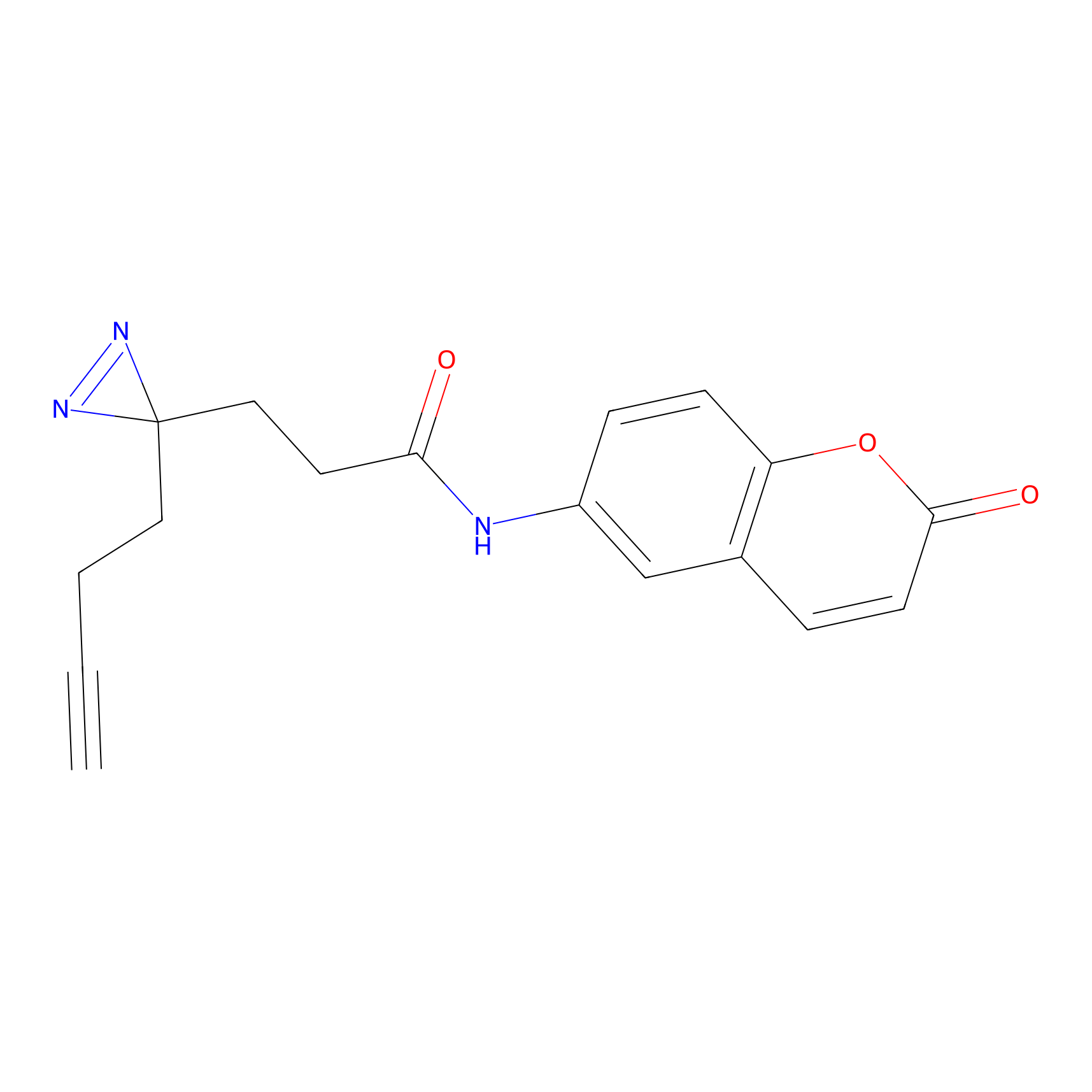 |
6.00 | LDD0465 | [11] | |
|
DA-2 Probe Info |
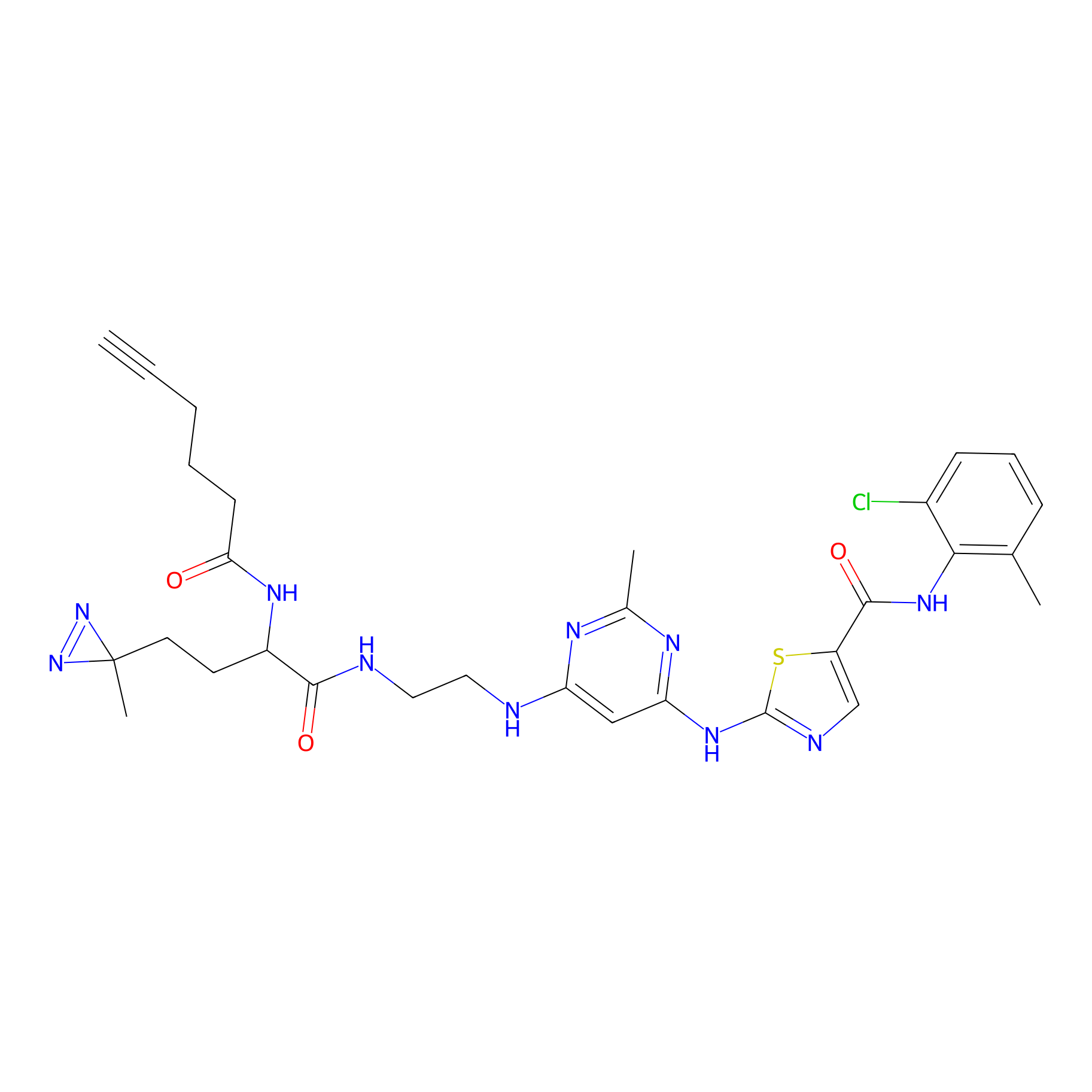 |
N.A. | LDD0070 | [12] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0519 | 1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)-2-nitroethan-1-one | MDA-MB-231 | C586(1.43) | LDD2112 | [2] |
| LDCM0524 | 2-Cyano-N-(2-morpholin-4-yl-ethyl)-acetamide | MDA-MB-231 | C586(0.56); C488(1.19) | LDD2117 | [2] |
| LDCM0558 | 2-Cyano-N-phenylacetamide | MDA-MB-231 | C586(1.36) | LDD2152 | [2] |
| LDCM0625 | F8 | Ramos | C586(0.95) | LDD2187 | [13] |
| LDCM0572 | Fragment10 | Ramos | C586(0.50) | LDD2189 | [13] |
| LDCM0573 | Fragment11 | Ramos | C586(2.19) | LDD2190 | [13] |
| LDCM0574 | Fragment12 | Ramos | C586(1.03) | LDD2191 | [13] |
| LDCM0576 | Fragment14 | Ramos | C586(1.22) | LDD2193 | [13] |
| LDCM0579 | Fragment20 | Ramos | C586(0.59) | LDD2194 | [13] |
| LDCM0582 | Fragment23 | Ramos | C586(0.69) | LDD2196 | [13] |
| LDCM0588 | Fragment30 | Ramos | C586(1.54) | LDD2199 | [13] |
| LDCM0590 | Fragment32 | Ramos | C586(0.69) | LDD2201 | [13] |
| LDCM0468 | Fragment33 | Ramos | C586(1.20) | LDD2202 | [13] |
| LDCM0596 | Fragment38 | Ramos | C586(1.37) | LDD2203 | [13] |
| LDCM0566 | Fragment4 | Ramos | C586(0.54) | LDD2184 | [13] |
| LDCM0569 | Fragment7 | Ramos | C586(0.75) | LDD2186 | [13] |
| LDCM0571 | Fragment9 | Ramos | C586(0.47) | LDD2188 | [13] |
| LDCM0022 | KB02 | Ramos | C586(0.58) | LDD2182 | [13] |
| LDCM0023 | KB03 | Jurkat | C572(6.40) | LDD0209 | [3] |
| LDCM0024 | KB05 | COLO792 | C526(2.72); C586(1.65) | LDD3310 | [14] |
| LDCM0506 | Nucleophilic fragment 16a | MDA-MB-231 | C586(1.23); C488(0.93) | LDD2099 | [2] |
| LDCM0514 | Nucleophilic fragment 20a | MDA-MB-231 | C586(1.44) | LDD2107 | [2] |
| LDCM0516 | Nucleophilic fragment 21a | MDA-MB-231 | C586(1.20) | LDD2109 | [2] |
| LDCM0518 | Nucleophilic fragment 22a | MDA-MB-231 | C586(1.70) | LDD2111 | [2] |
| LDCM0526 | Nucleophilic fragment 26a | MDA-MB-231 | C586(2.43) | LDD2119 | [2] |
| LDCM0530 | Nucleophilic fragment 28a | MDA-MB-231 | C586(1.14); C488(1.31) | LDD2123 | [2] |
| LDCM0532 | Nucleophilic fragment 29a | MDA-MB-231 | C586(1.13); C488(1.00) | LDD2125 | [2] |
| LDCM0534 | Nucleophilic fragment 30a | MDA-MB-231 | C586(1.23) | LDD2127 | [2] |
| LDCM0536 | Nucleophilic fragment 31 | MDA-MB-231 | C586(1.01) | LDD2129 | [2] |
| LDCM0542 | Nucleophilic fragment 37 | MDA-MB-231 | C586(1.62); C488(0.83) | LDD2135 | [2] |
| LDCM0543 | Nucleophilic fragment 38 | MDA-MB-231 | C586(1.60); C488(1.75) | LDD2136 | [2] |
| LDCM0544 | Nucleophilic fragment 39 | MDA-MB-231 | C586(1.38) | LDD2137 | [2] |
| LDCM0546 | Nucleophilic fragment 40 | MDA-MB-231 | C586(1.06) | LDD2140 | [2] |
| LDCM0550 | Nucleophilic fragment 5a | MDA-MB-231 | C488(2.24) | LDD2144 | [2] |
| LDCM0552 | Nucleophilic fragment 6a | MDA-MB-231 | C586(1.09) | LDD2146 | [2] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Enzyme
Other
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| SHC SH2 domain-binding protein 1 (SHCBP1) | . | Q8NEM2 | |||
| TERF1-interacting nuclear factor 2 (TINF2) | . | Q9BSI4 | |||
The Drug(s) Related To This Target
Approved
Phase 3
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Enzastaurin | Small molecular drug | D0I6VU | |||
| Ruboxistaurin Hydrochloride | . | D0MV4W | |||
Phase 2
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Ly333531 | Small molecular drug | D0W3LI | |||
| Sotrastaurin Acetate | Small molecular drug | D0F5HB | |||
Phase 1
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Ms-553 | . | D98VPM | |||
Investigative
Discontinued
| Drug Name | Drug Type | External ID | |||
|---|---|---|---|---|---|
| Balanol | Small molecular drug | D0R2TM | |||
| Linetastine | Small molecular drug | D0E8UV | |||
| Ly-317644 | Small molecular drug | D0X0HN | |||
| Ro-320432 | Small molecular drug | D0R5ZR | |||
References
