Details of the Target
General Information of Target
| Target ID | LDTP05040 | |||||
|---|---|---|---|---|---|---|
| Target Name | Guanine nucleotide-binding protein G(s) subunit alpha isoforms short (GNAS) | |||||
| Gene Name | GNAS | |||||
| Gene ID | 2778 | |||||
| Synonyms |
GNAS1; GSP; Guanine nucleotide-binding protein G(s) subunit alpha isoforms short; Adenylate cyclase-stimulating G alpha protein |
|||||
| 3D Structure | ||||||
| Sequence |
MGCLGNSKTEDQRNEEKAQREANKKIEKQLQKDKQVYRATHRLLLLGAGESGKSTIVKQM
RILHVNGFNGEGGEEDPQAARSNSDGEKATKVQDIKNNLKEAIETIVAAMSNLVPPVELA NPENQFRVDYILSVMNVPDFDFPPEFYEHAKALWEDEGVRACYERSNEYQLIDCAQYFLD KIDVIKQADYVPSDQDLLRCRVLTSGIFETKFQVDKVNFHMFDVGGQRDERRKWIQCFND VTAIIFVVASSSYNMVIREDNQTNRLQEALNLFKSIWNNRWLRTISVILFLNKQDLLAEK VLAGKSKIEDYFPEFARYTTPEDATPEPGEDPRVTRAKYFIRDEFLRISTASGDGRHYCY PHFTCAVDTENIRRVFNDCRDIIQRMHLRQYELL |
|||||
| Target Bioclass |
Transporter and channel
|
|||||
| Family |
G-alpha family, G(s) subfamily
|
|||||
| Subcellular location |
Cell membrane
|
|||||
| Function |
Guanine nucleotide-binding proteins (G proteins) function as transducers in numerous signaling pathways controlled by G protein-coupled receptors (GPCRs). Signaling involves the activation of adenylyl cyclases, resulting in increased levels of the signaling molecule cAMP. GNAS functions downstream of several GPCRs, including beta-adrenergic receptors. Stimulates the Ras signaling pathway via RAPGEF2.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
| ChEMBL ID | ||||||
Target Site Mutations in Different Cell Lines
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
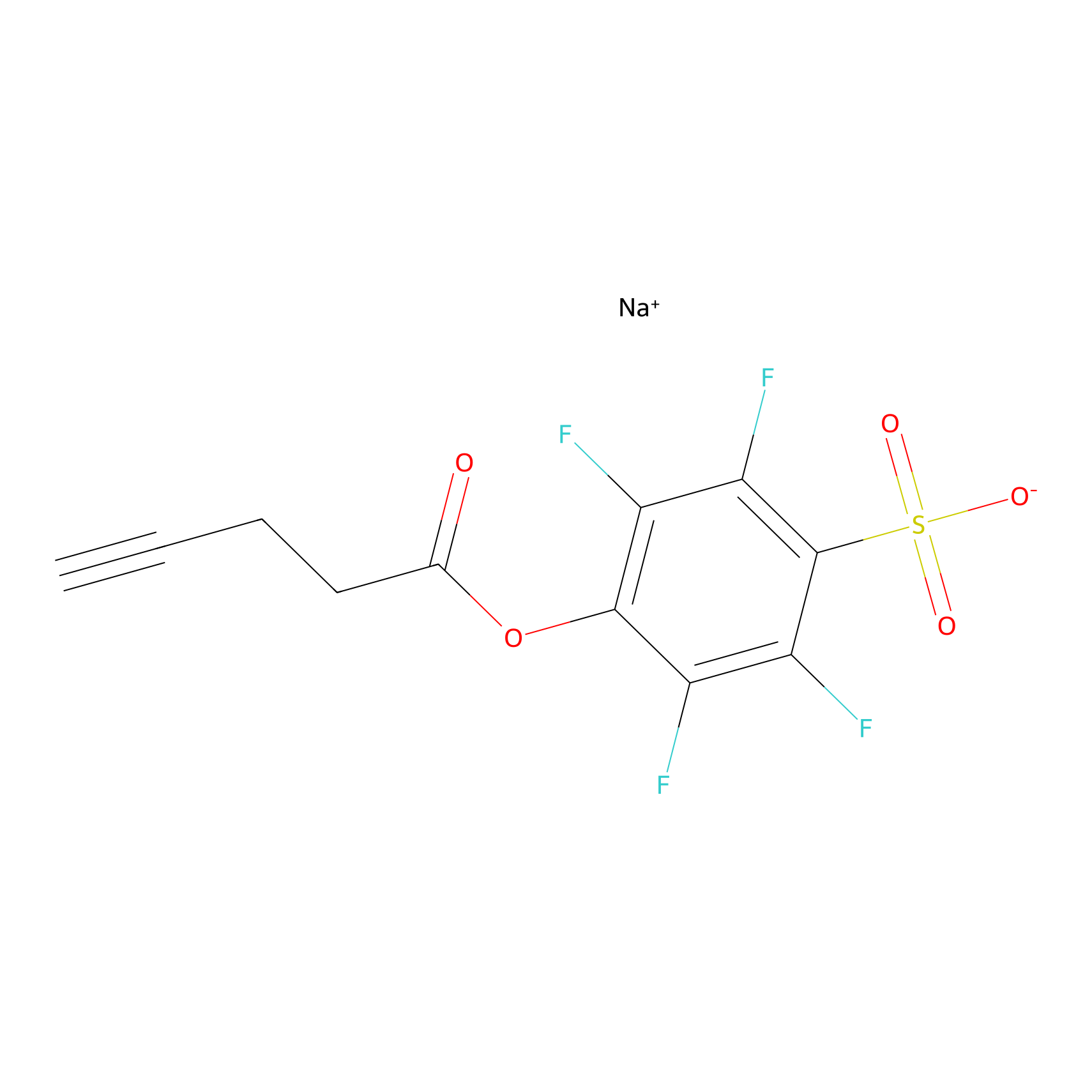
|
m-APA Probe Info |
 |
13.10 | LDD0402 | [1] | |
|
CHEMBL5175495 Probe Info |
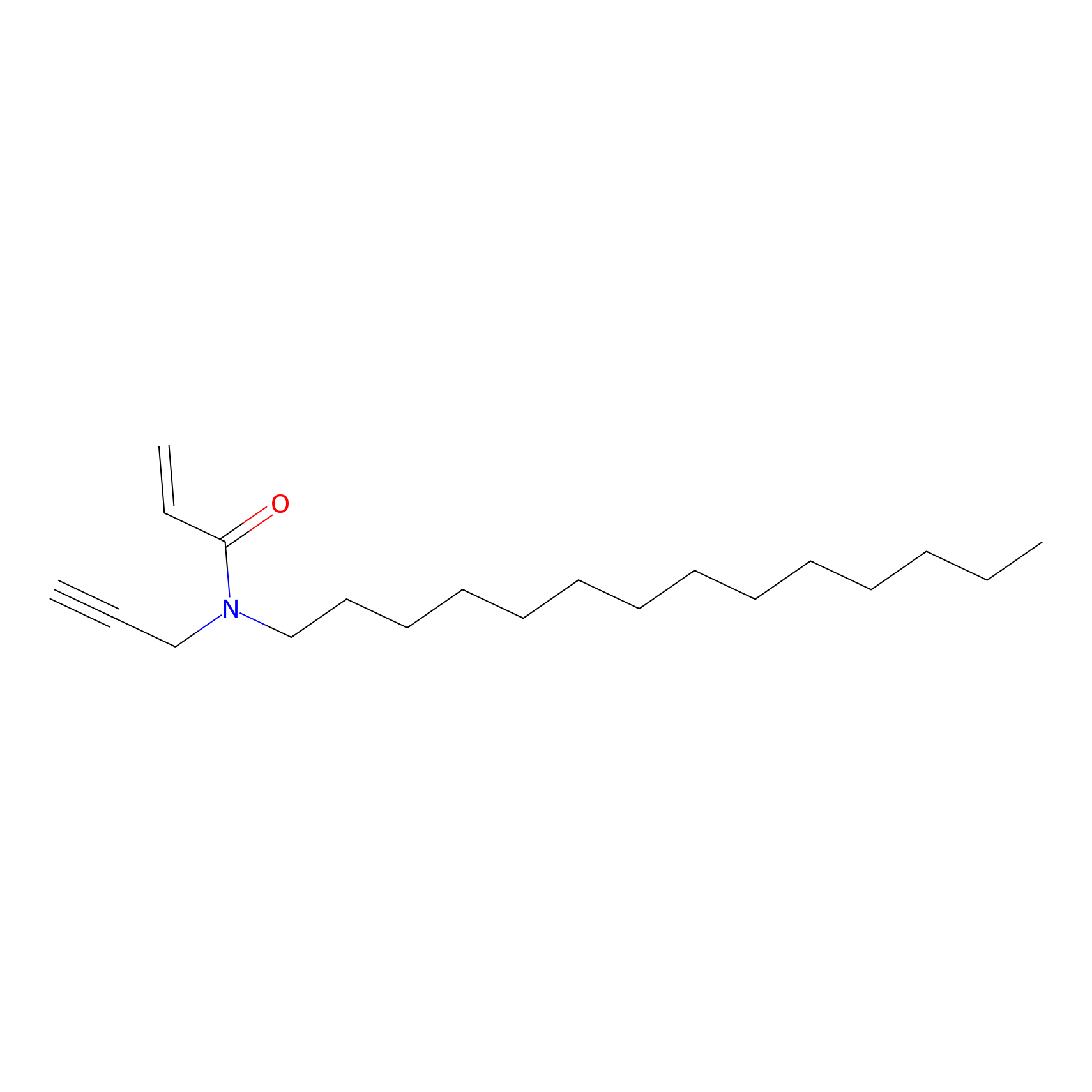 |
7.01 | LDD0196 | [2] | |
|
TH211 Probe Info |
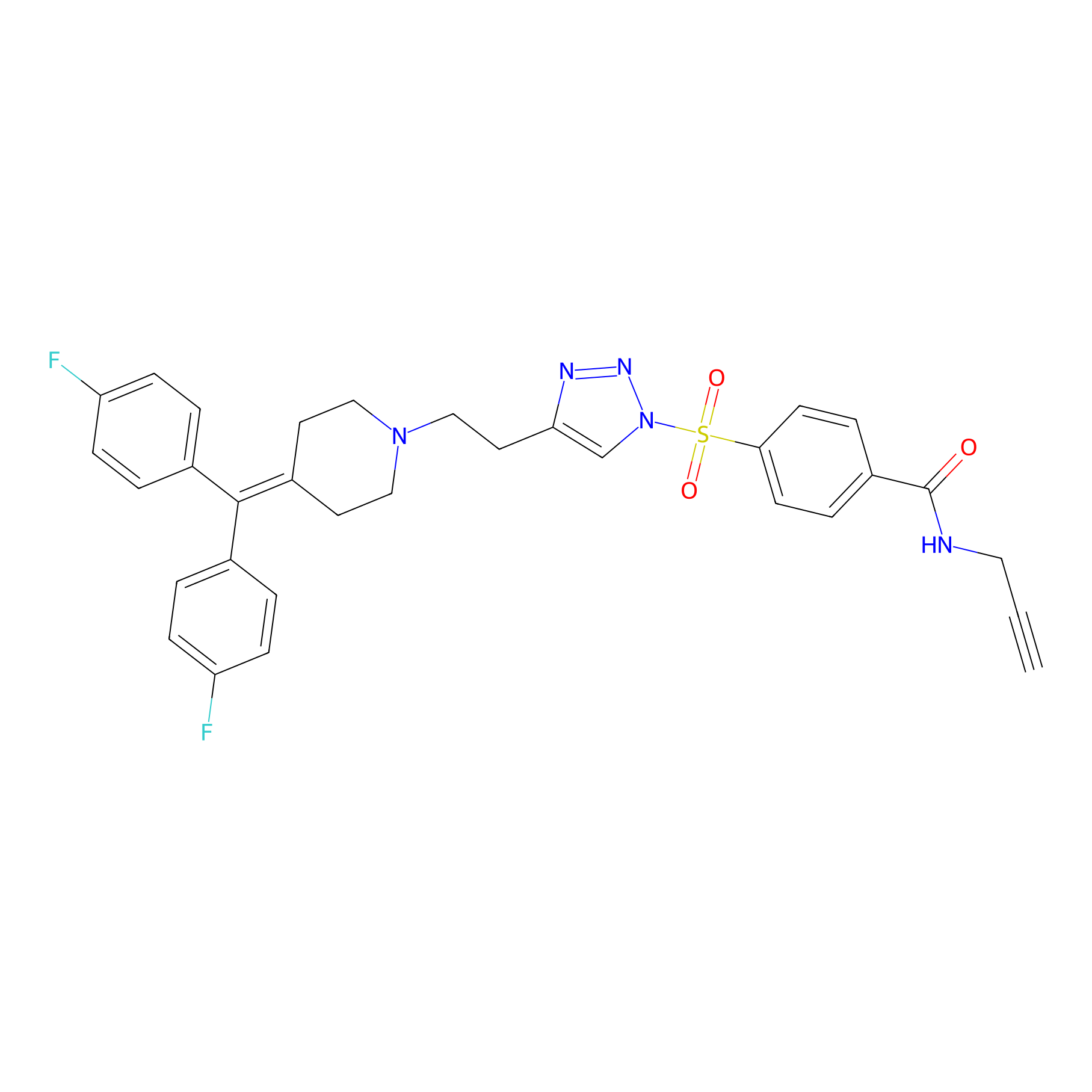 |
Y318(20.00); Y311(10.47); Y360(6.40) | LDD0260 | [3] | |
|
STPyne Probe Info |
 |
K338(6.25); K34(7.66) | LDD0277 | [4] | |
|
BTD Probe Info |
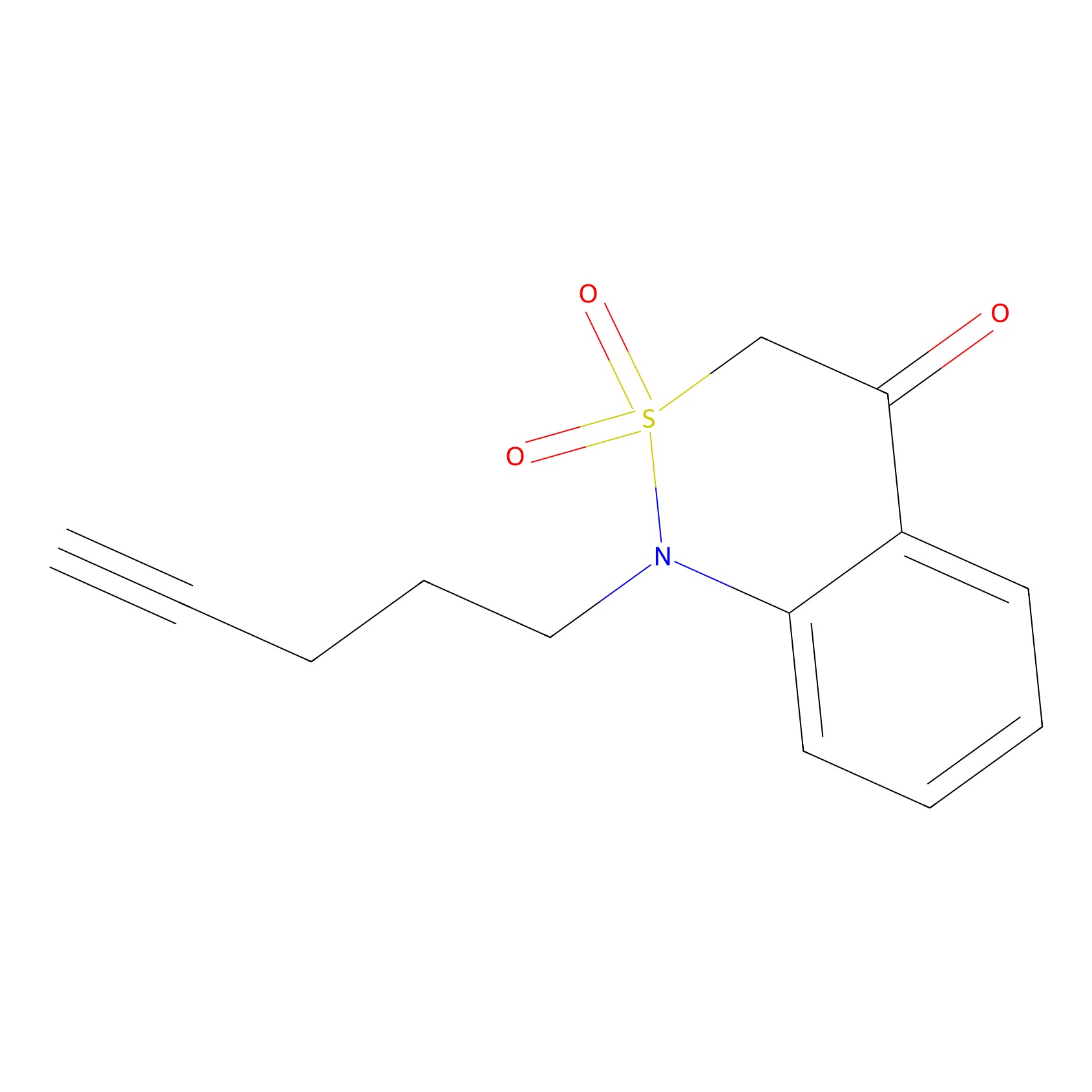 |
C3(6.39) | LDD1699 | [5] | |
|
Probe 1 Probe Info |
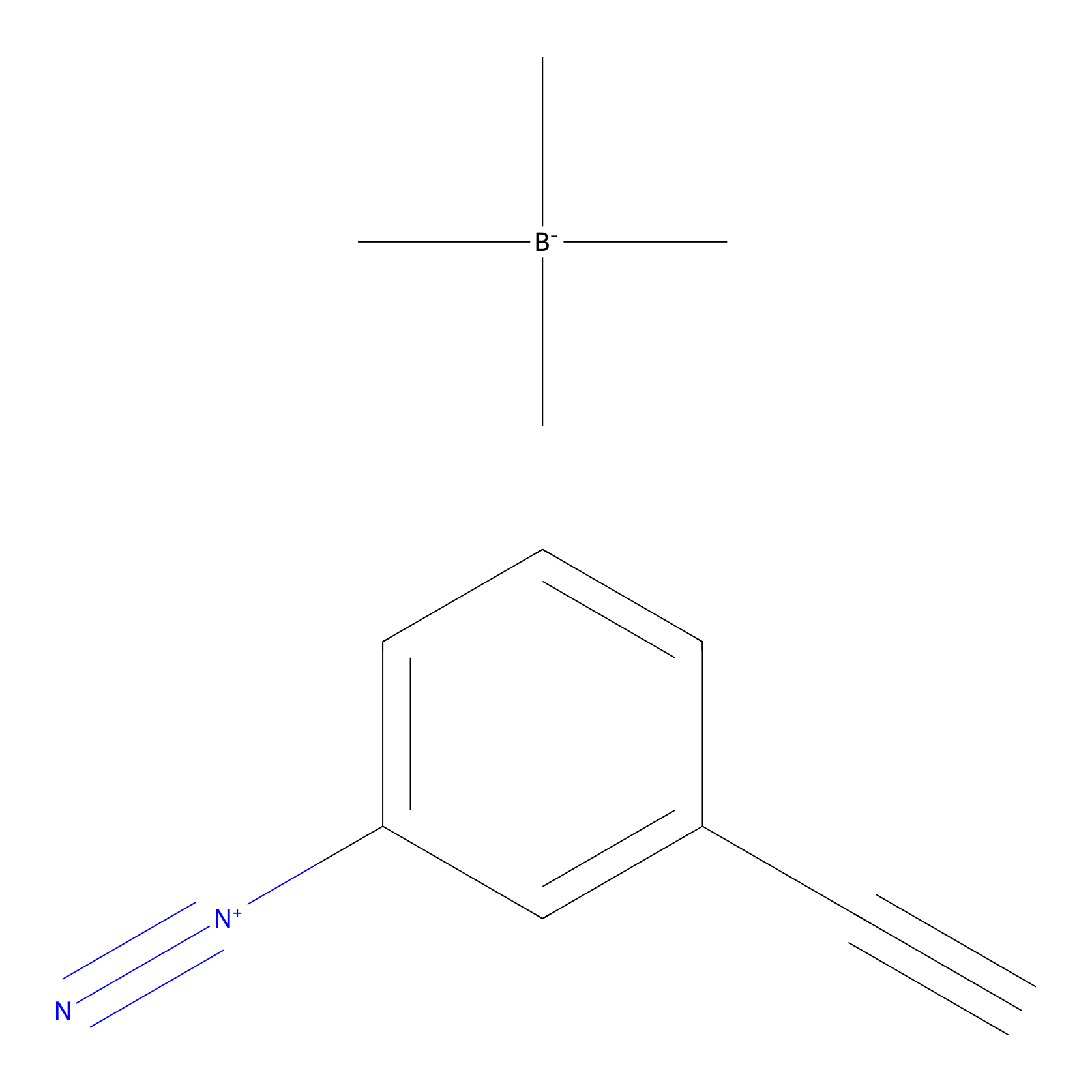 |
Y190(36.13); Y311(10.86); Y318(17.98); Y339(28.04) | LDD3495 | [6] | |
|
ATP probe Probe Info |
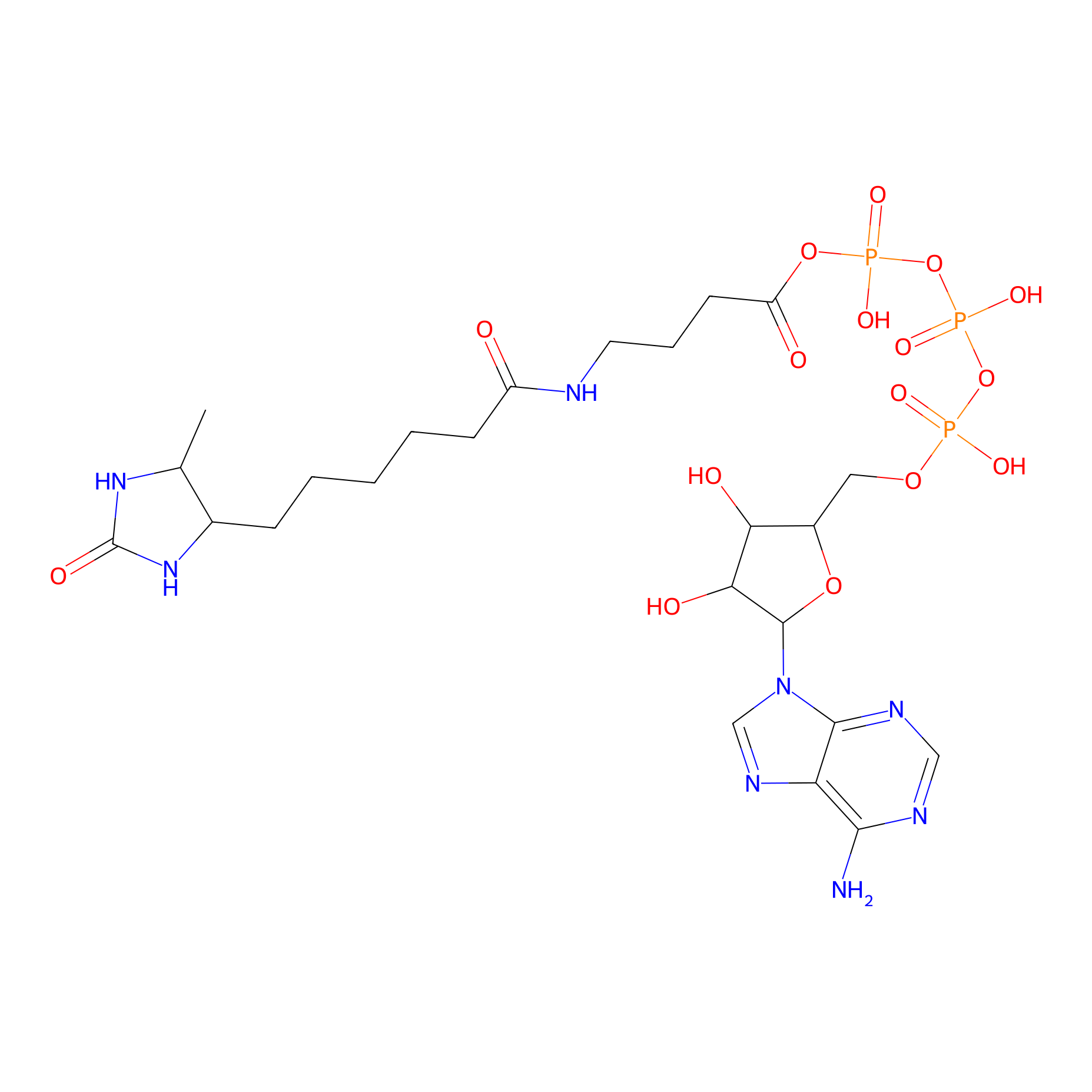 |
K211(0.00); K216(0.00) | LDD0199 | [7] | |
|
4-Iodoacetamidophenylacetylene Probe Info |
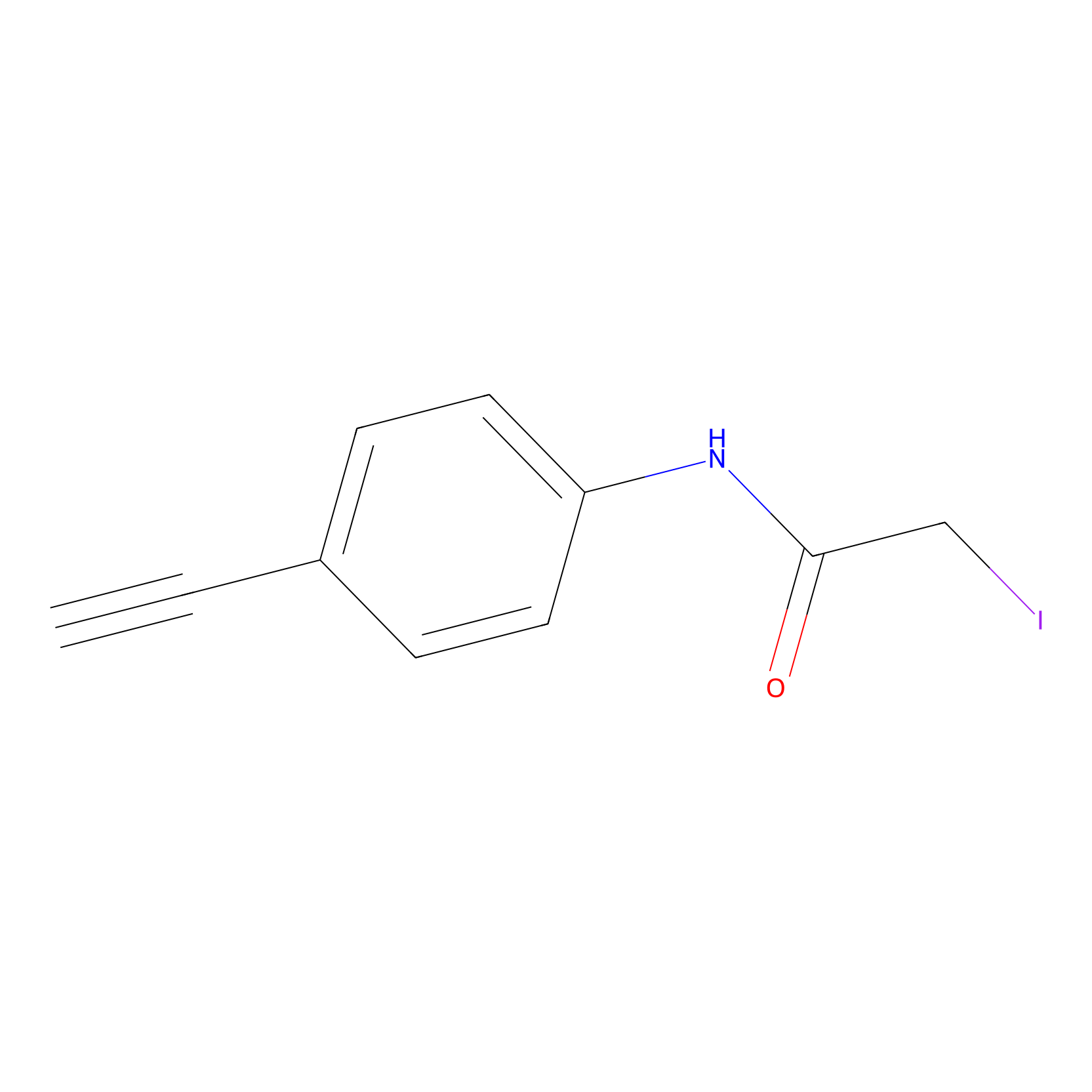 |
C3(0.00); C379(0.00); C174(0.00) | LDD0038 | [8] | |
|
IA-alkyne Probe Info |
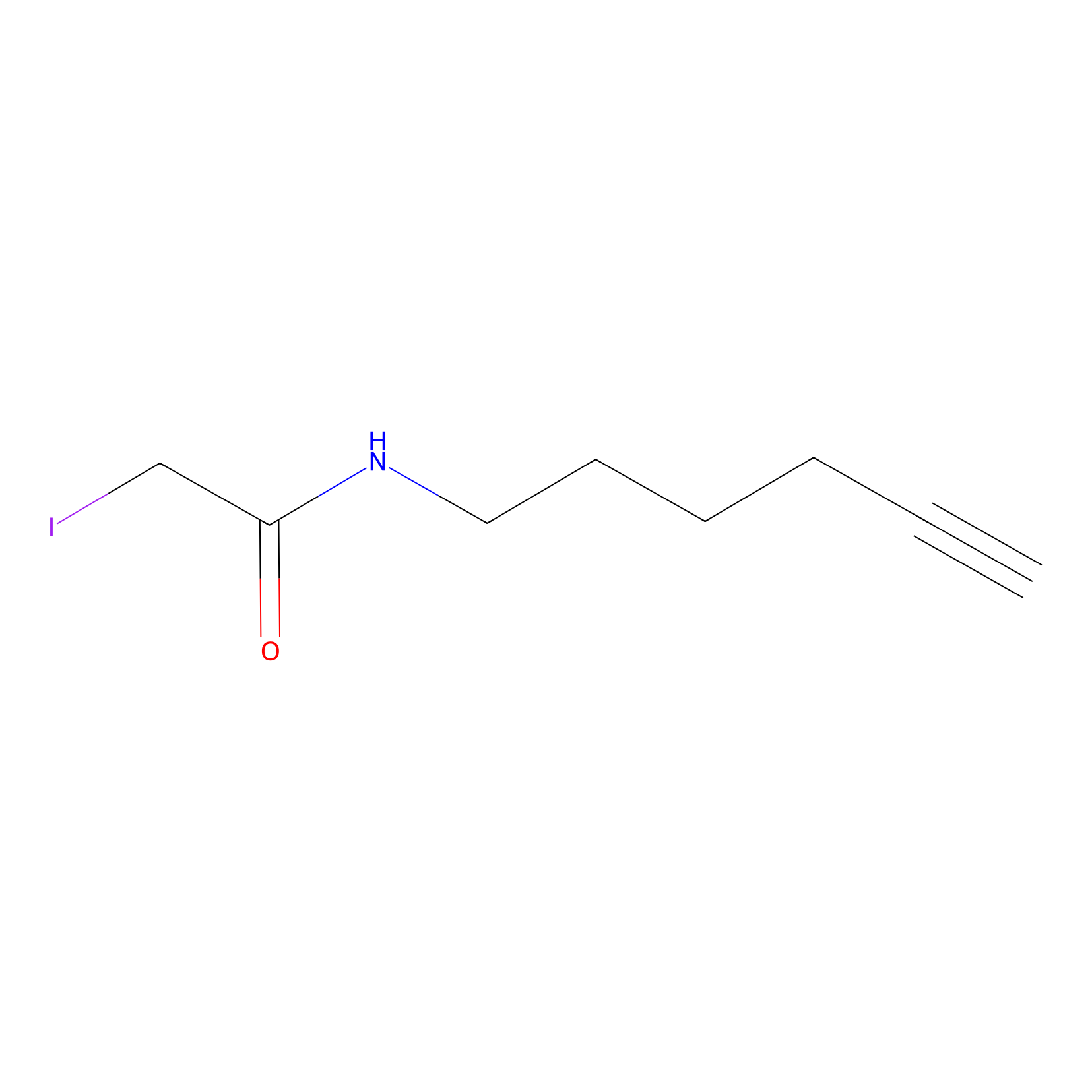 |
C379(0.00); C174(0.00); C3(0.00); C359(0.00) | LDD0036 | [8] | |
|
Lodoacetamide azide Probe Info |
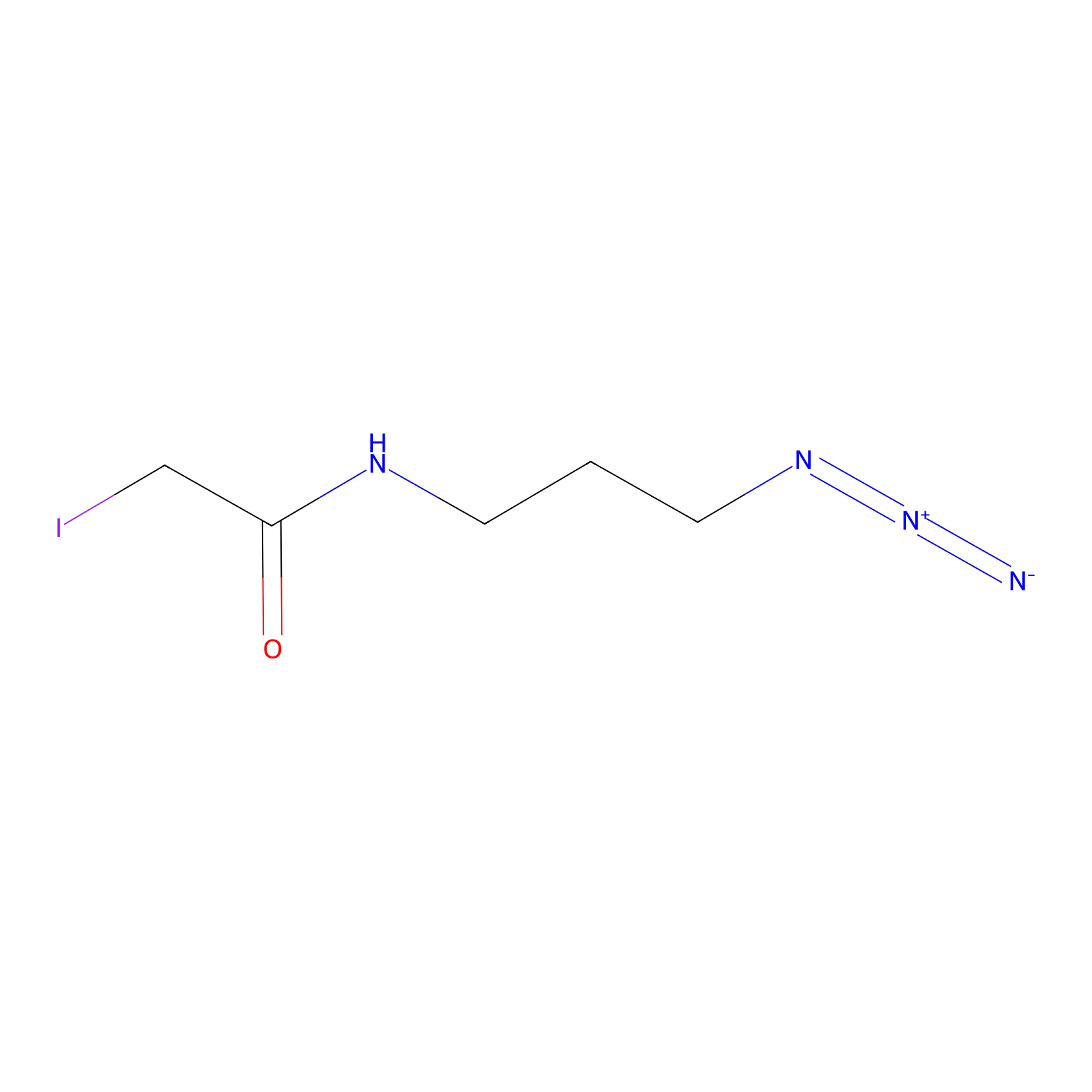 |
C3(0.00); C379(0.00); C174(0.00); C359(0.00) | LDD0037 | [8] | |
|
JW-RF-010 Probe Info |
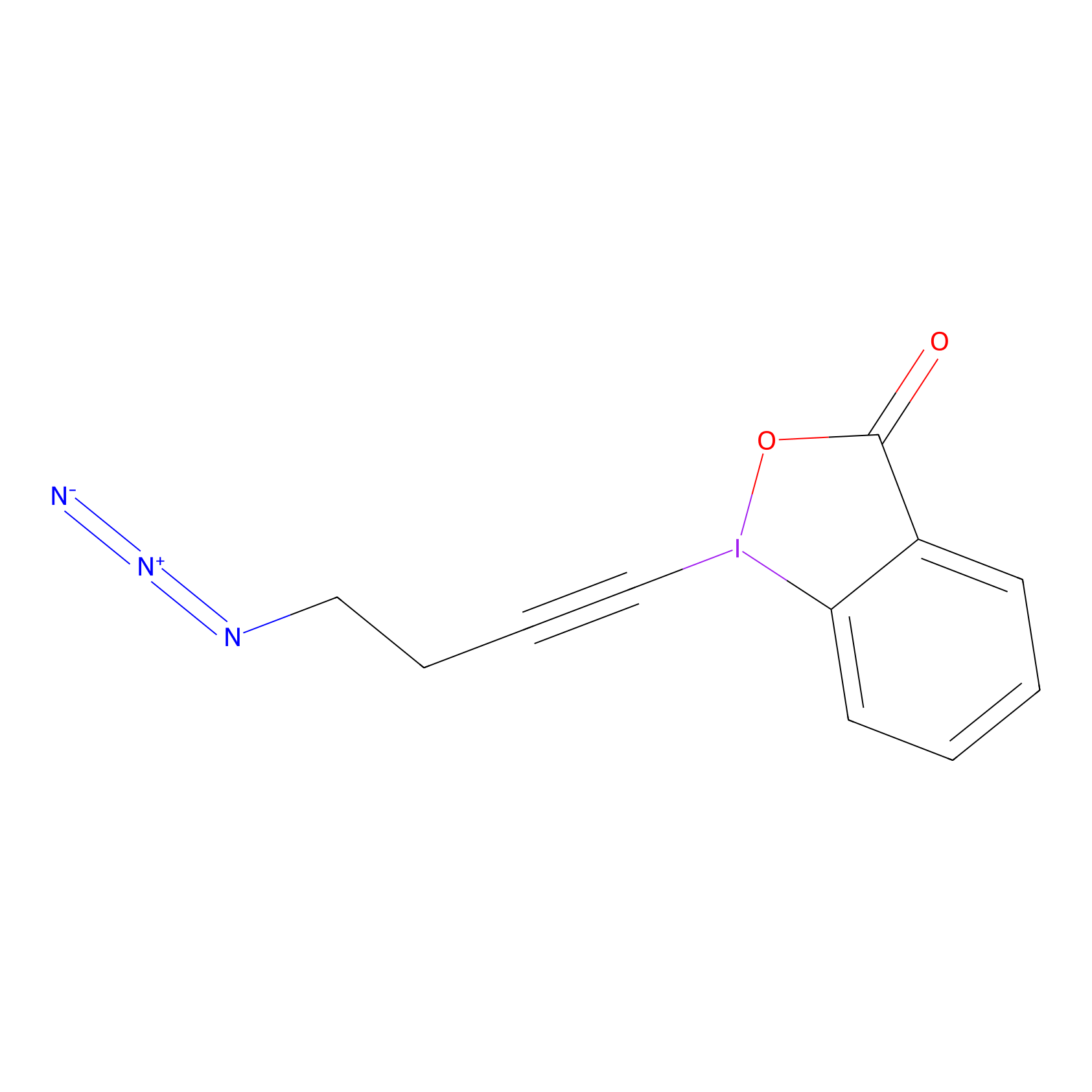 |
N.A. | LDD0026 | [9] | |
|
TFBX Probe Info |
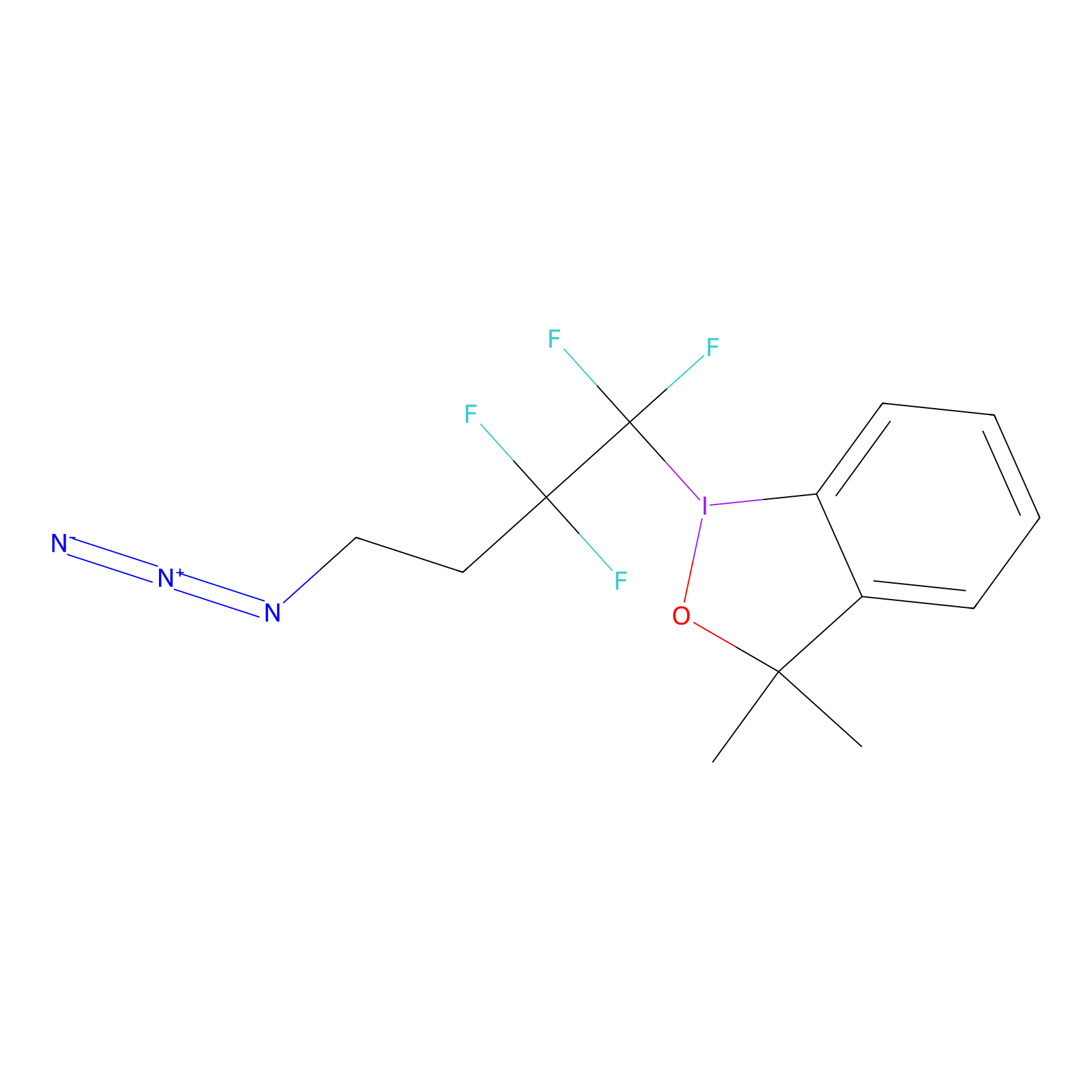 |
C3(0.00); C379(0.00) | LDD0027 | [9] | |
|
IPM Probe Info |
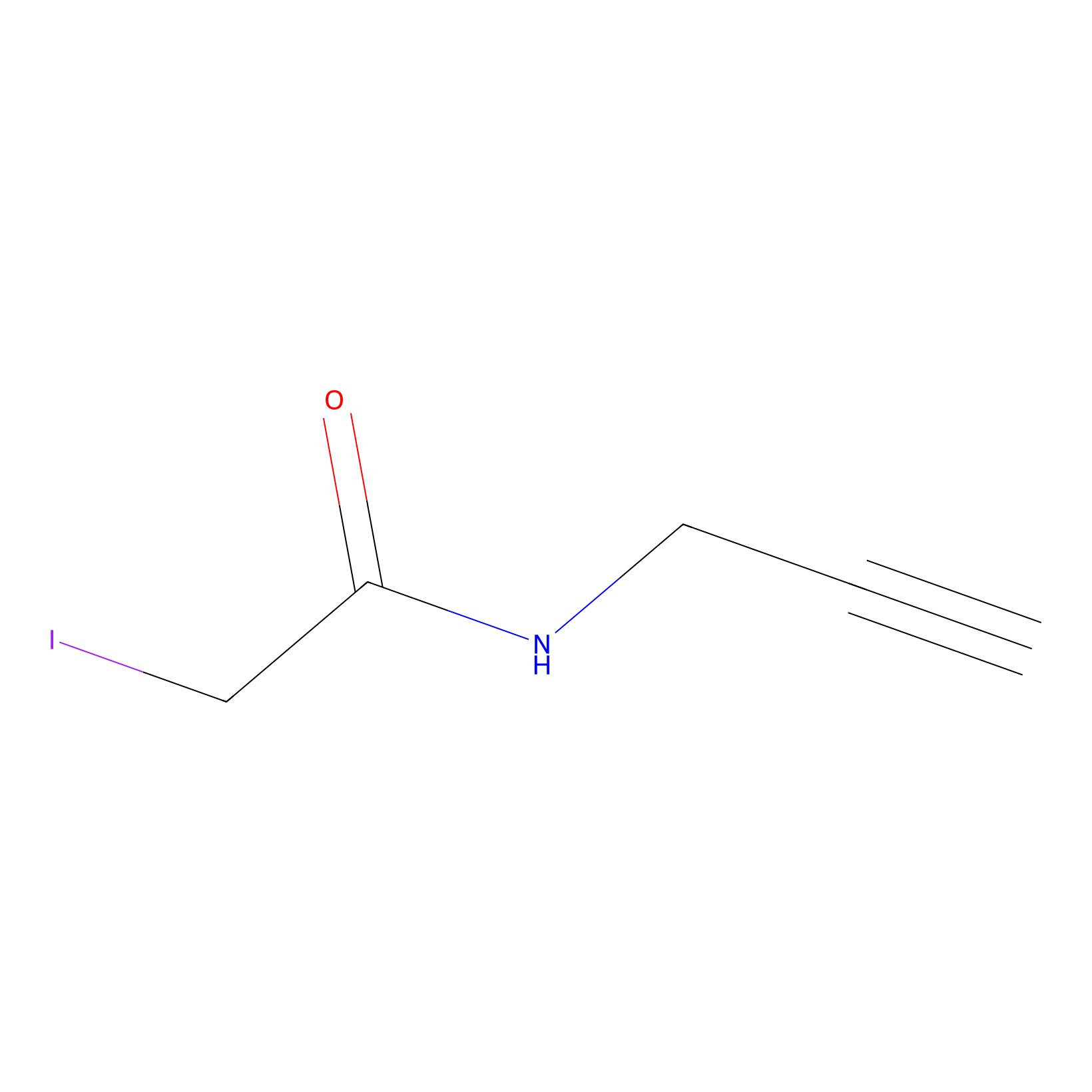 |
N.A. | LDD0005 | [10] | |
|
Phosphinate-6 Probe Info |
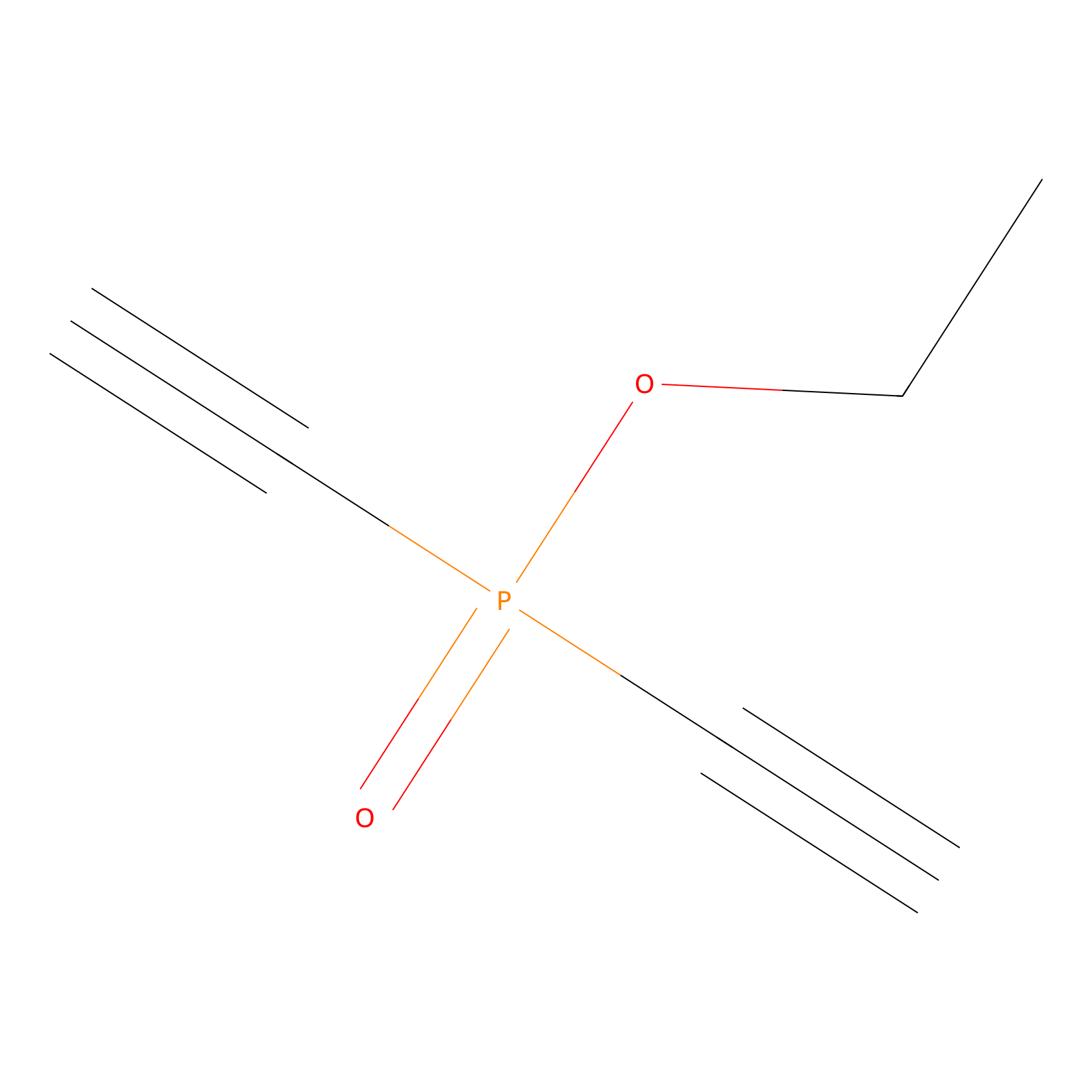 |
C359(0.00); C3(0.00) | LDD0018 | [11] | |
|
AOyne Probe Info |
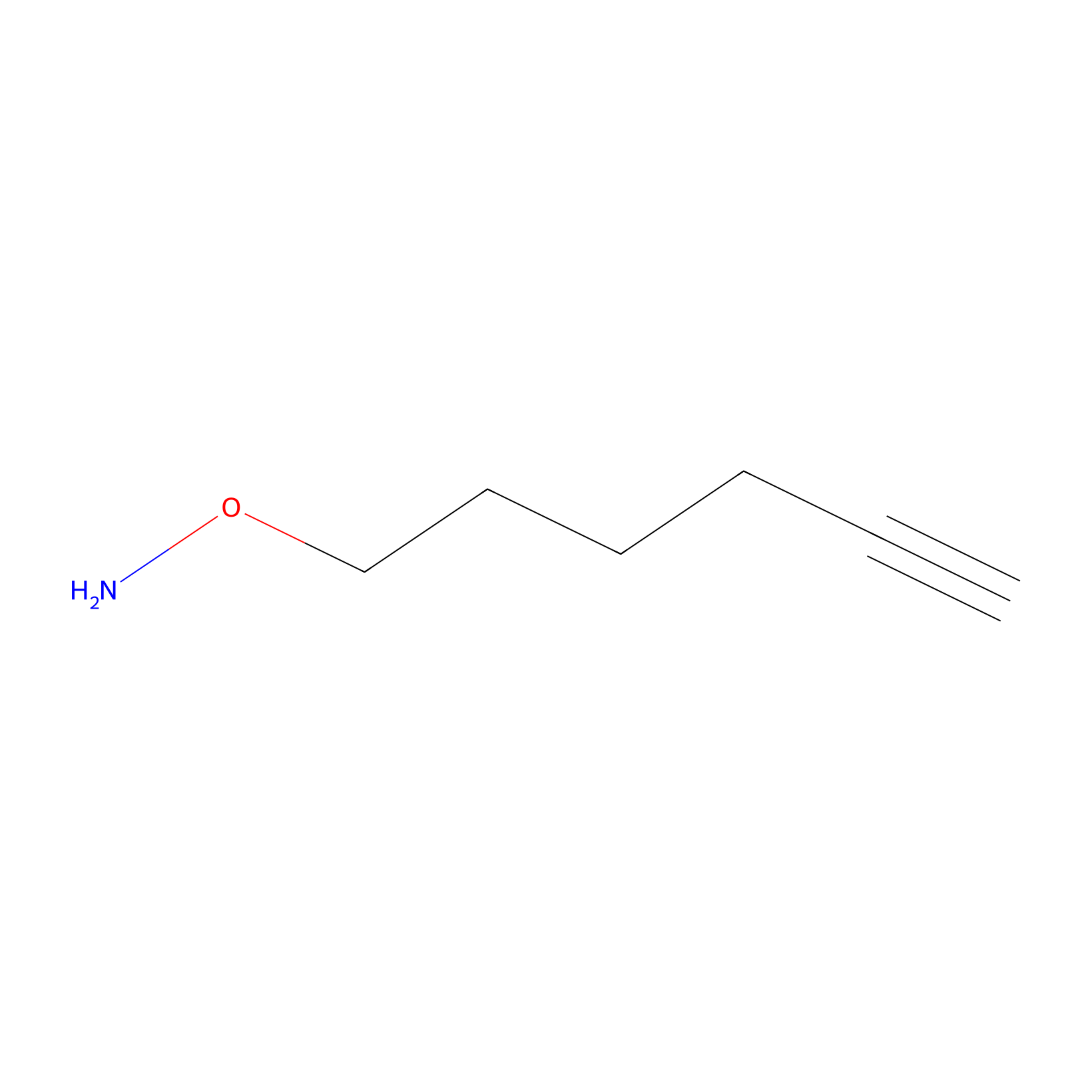 |
15.00 | LDD0443 | [12] | |
|
NAIA_5 Probe Info |
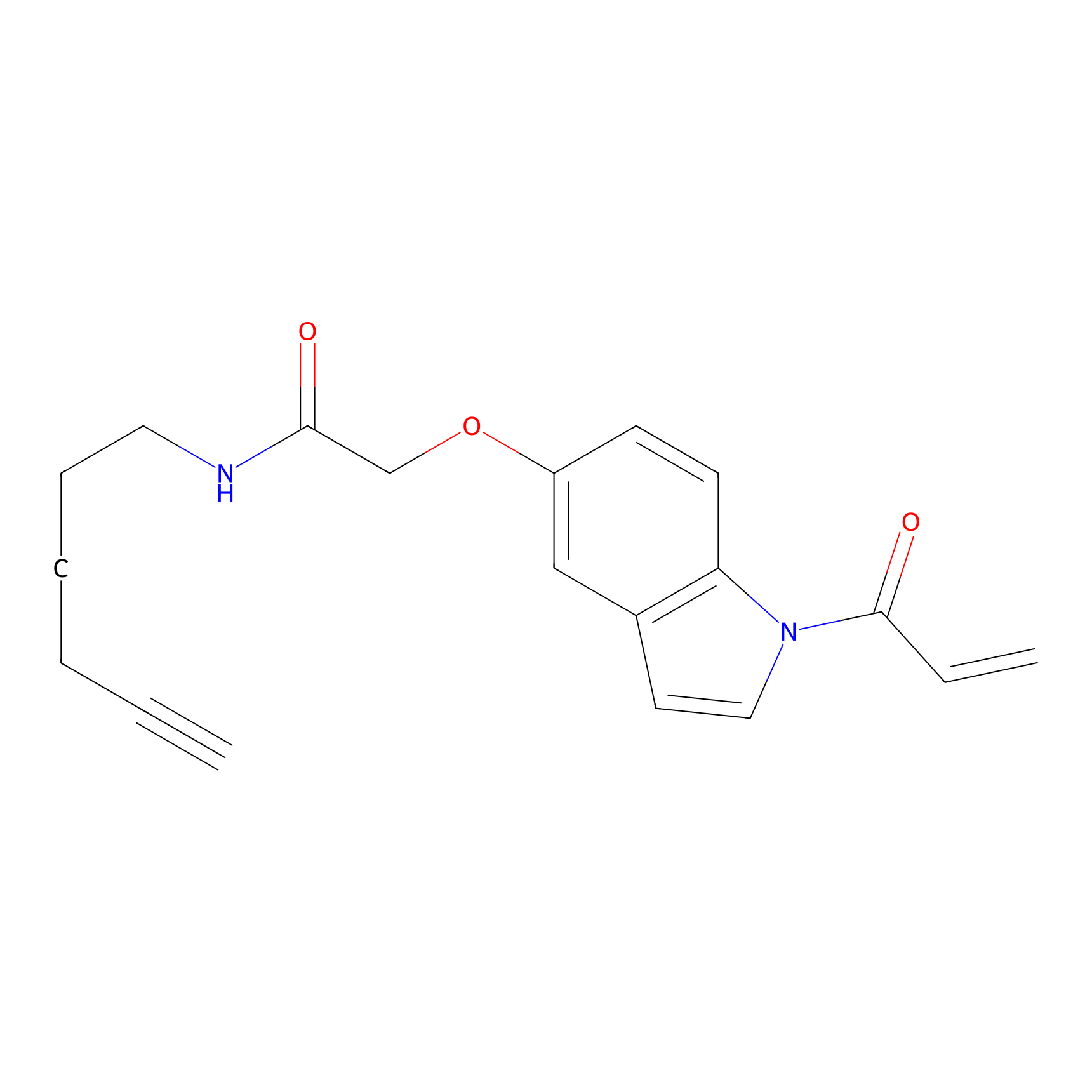 |
C162(0.00); C174(0.00); C237(0.00); C365(0.00) | LDD2223 | [13] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
STS-2 Probe Info |
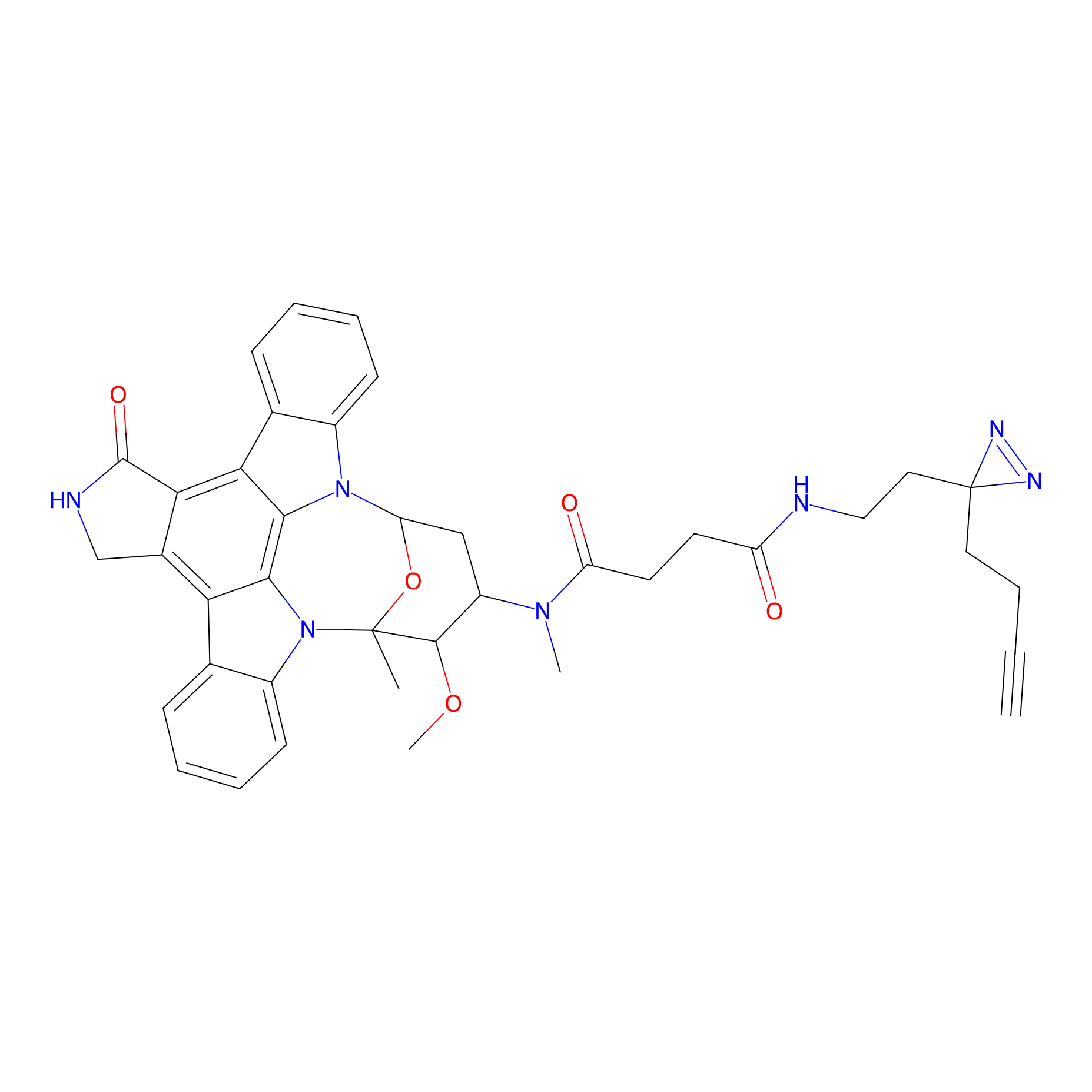 |
N.A. | LDD0139 | [14] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0548 | 1-(4-(Benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)-2-nitroethan-1-one | MDA-MB-231 | C3(1.02) | LDD2142 | [5] |
| LDCM0519 | 1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)-2-nitroethan-1-one | MDA-MB-231 | C3(1.21) | LDD2112 | [5] |
| LDCM0502 | 1-(Cyanoacetyl)piperidine | MDA-MB-231 | C3(0.90) | LDD2095 | [5] |
| LDCM0537 | 2-Cyano-N,N-dimethylacetamide | MDA-MB-231 | C3(1.09) | LDD2130 | [5] |
| LDCM0524 | 2-Cyano-N-(2-morpholin-4-yl-ethyl)-acetamide | MDA-MB-231 | C3(0.99) | LDD2117 | [5] |
| LDCM0539 | 3-(4-Isopropylpiperazin-1-yl)-3-oxopropanenitrile | MDA-MB-231 | C3(0.66) | LDD2132 | [5] |
| LDCM0538 | 4-(Cyanoacetyl)morpholine | MDA-MB-231 | C3(0.89) | LDD2131 | [5] |
| LDCM0545 | Acetamide | MDA-MB-231 | C3(0.90) | LDD2138 | [5] |
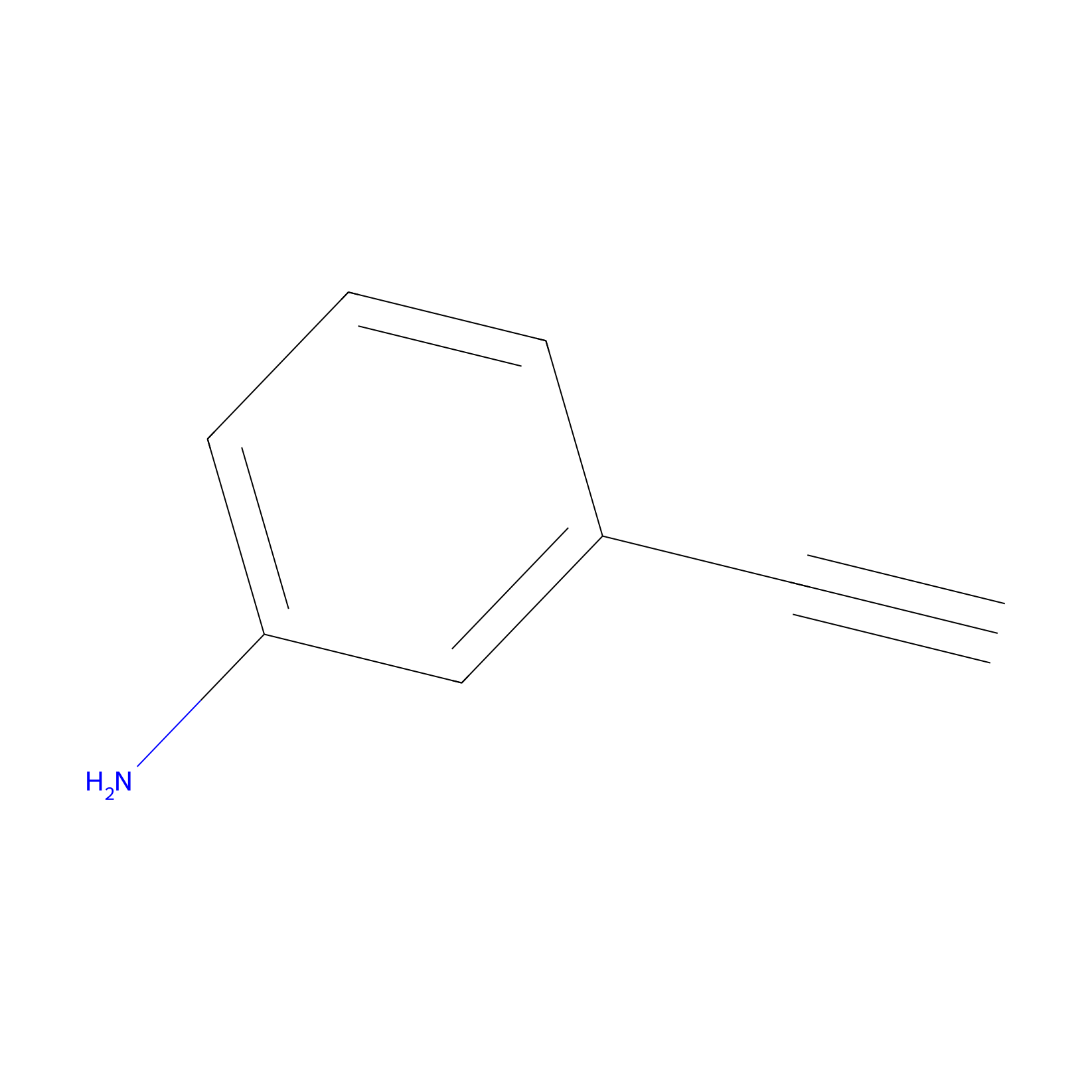
| LDCM0156 | Aniline | NCI-H1299 | 15.00 | LDD0403 | [1] |
| LDCM0498 | BS-3668 | MDA-MB-231 | C3(0.51) | LDD2091 | [5] |
| LDCM0213 | Electrophilic fragment 2 | MDA-MB-231 | C3(3.30); C379(1.54) | LDD1702 | [5] |
| LDCM0625 | F8 | Ramos | C3(1.24); C379(0.56); 1.02 | LDD2187 | [15] |
| LDCM0572 | Fragment10 | Ramos | C3(1.78); C379(0.55) | LDD2189 | [15] |
| LDCM0573 | Fragment11 | Ramos | C3(4.23); C379(0.49) | LDD2190 | [15] |
| LDCM0574 | Fragment12 | Ramos | C3(0.86); C379(0.59) | LDD2191 | [15] |
| LDCM0575 | Fragment13 | Ramos | C3(0.97); C379(0.84) | LDD2192 | [15] |
| LDCM0576 | Fragment14 | Ramos | C3(0.72); C379(0.35); 0.84 | LDD2193 | [15] |
| LDCM0579 | Fragment20 | Ramos | C3(1.24); C379(0.55) | LDD2194 | [15] |
| LDCM0580 | Fragment21 | Ramos | C3(0.98); C379(0.72) | LDD2195 | [15] |
| LDCM0582 | Fragment23 | Ramos | C3(0.75); C379(1.40) | LDD2196 | [15] |
| LDCM0578 | Fragment27 | Ramos | C3(0.92); C379(0.88) | LDD2197 | [15] |
| LDCM0586 | Fragment28 | Ramos | C3(0.76); C379(0.88) | LDD2198 | [15] |
| LDCM0588 | Fragment30 | Ramos | C3(1.41); C379(0.96) | LDD2199 | [15] |
| LDCM0589 | Fragment31 | Ramos | C3(0.83); C379(0.87) | LDD2200 | [15] |
| LDCM0590 | Fragment32 | Ramos | C3(2.20); C379(0.52) | LDD2201 | [15] |
| LDCM0468 | Fragment33 | Ramos | C3(1.11); C379(0.66) | LDD2202 | [15] |
| LDCM0596 | Fragment38 | Ramos | C3(1.04); C379(0.83) | LDD2203 | [15] |
| LDCM0566 | Fragment4 | Ramos | C3(1.13); C379(0.67) | LDD2184 | [15] |
| LDCM0610 | Fragment52 | Ramos | C3(1.14); C379(1.28) | LDD2204 | [15] |
| LDCM0614 | Fragment56 | Ramos | C3(2.27); C379(1.10) | LDD2205 | [15] |
| LDCM0569 | Fragment7 | Ramos | C3(1.23); C379(0.91); 0.76 | LDD2186 | [15] |
| LDCM0571 | Fragment9 | Ramos | C3(1.16); C379(0.66) | LDD2188 | [15] |
| LDCM0022 | KB02 | Ramos | C3(3.58); C379(0.97) | LDD2182 | [15] |
| LDCM0023 | KB03 | MDA-MB-231 | C379(10.92); C3(5.66) | LDD1701 | [5] |
| LDCM0024 | KB05 | Ramos | C3(1.91); C379(0.84) | LDD2185 | [15] |
| LDCM0509 | N-(4-bromo-3,5-dimethylphenyl)-2-nitroacetamide | MDA-MB-231 | C3(0.89) | LDD2102 | [5] |
| LDCM0528 | N-(4-bromophenyl)-2-cyano-N-phenylacetamide | MDA-MB-231 | C3(0.78) | LDD2121 | [5] |
| LDCM0496 | Nucleophilic fragment 11a | MDA-MB-231 | C3(0.74) | LDD2089 | [5] |
| LDCM0497 | Nucleophilic fragment 11b | MDA-MB-231 | C379(0.65) | LDD2090 | [5] |
| LDCM0499 | Nucleophilic fragment 12b | MDA-MB-231 | C3(0.96) | LDD2092 | [5] |
| LDCM0501 | Nucleophilic fragment 13b | MDA-MB-231 | C3(1.27) | LDD2094 | [5] |
| LDCM0505 | Nucleophilic fragment 15b | MDA-MB-231 | C3(1.14) | LDD2098 | [5] |
| LDCM0506 | Nucleophilic fragment 16a | MDA-MB-231 | C3(0.93) | LDD2099 | [5] |
| LDCM0507 | Nucleophilic fragment 16b | MDA-MB-231 | C3(1.34) | LDD2100 | [5] |
| LDCM0511 | Nucleophilic fragment 18b | MDA-MB-231 | C3(1.06) | LDD2104 | [5] |
| LDCM0512 | Nucleophilic fragment 19a | MDA-MB-231 | C3(1.77) | LDD2105 | [5] |
| LDCM0514 | Nucleophilic fragment 20a | MDA-MB-231 | C379(1.31) | LDD2107 | [5] |
| LDCM0515 | Nucleophilic fragment 20b | MDA-MB-231 | C3(1.03) | LDD2108 | [5] |
| LDCM0521 | Nucleophilic fragment 23b | MDA-MB-231 | C3(0.87) | LDD2114 | [5] |
| LDCM0527 | Nucleophilic fragment 26b | MDA-MB-231 | C3(0.90) | LDD2120 | [5] |
| LDCM0531 | Nucleophilic fragment 28b | MDA-MB-231 | C3(1.11) | LDD2124 | [5] |
| LDCM0532 | Nucleophilic fragment 29a | MDA-MB-231 | C3(0.94) | LDD2125 | [5] |
| LDCM0533 | Nucleophilic fragment 29b | MDA-MB-231 | C3(1.36) | LDD2126 | [5] |
| LDCM0535 | Nucleophilic fragment 30b | MDA-MB-231 | C3(1.01); C379(0.95) | LDD2128 | [5] |
| LDCM0542 | Nucleophilic fragment 37 | MDA-MB-231 | C3(0.94) | LDD2135 | [5] |
| LDCM0543 | Nucleophilic fragment 38 | MDA-MB-231 | C3(1.23) | LDD2136 | [5] |
| LDCM0211 | Nucleophilic fragment 3b | MDA-MB-231 | C3(1.33) | LDD1700 | [5] |
| LDCM0546 | Nucleophilic fragment 40 | MDA-MB-231 | C3(1.24) | LDD2140 | [5] |
| LDCM0547 | Nucleophilic fragment 41 | MDA-MB-231 | C3(0.91) | LDD2141 | [5] |
| LDCM0549 | Nucleophilic fragment 43 | MDA-MB-231 | C3(1.01) | LDD2143 | [5] |
| LDCM0551 | Nucleophilic fragment 5b | MDA-MB-231 | C3(8.20) | LDD2145 | [5] |
| LDCM0552 | Nucleophilic fragment 6a | MDA-MB-231 | C3(0.98) | LDD2146 | [5] |
| LDCM0553 | Nucleophilic fragment 6b | MDA-MB-231 | C3(2.11) | LDD2147 | [5] |
| LDCM0556 | Nucleophilic fragment 8a | MDA-MB-231 | C3(0.52) | LDD2150 | [5] |
| LDCM0627 | NUDT7-COV-1 | HEK-293T | C3(0.69) | LDD2206 | [16] |
| LDCM0628 | OTUB2-COV-1 | HEK-293T | C3(0.74) | LDD2207 | [16] |
The Interaction Atlas With This Target
References
