| Synonyms |
Olaparib; 763113-22-0; AZD2281; Lynparza; AZD-2281; KU-0059436; AZD 2281; 1-(Cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoyl]piperazine; OLAPARIB cpd; 4-(3-(4-(Cyclopropanecarbonyl)piperazine-1-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; KU-59436; UNII-WOH1JD9AR8; WOH1JD9AR8; Olaparib (AZD-2281); Olaparib (AZD2281, Ku-0059436); Olaparibum; Olaparib [USAN:INN]; AZ2281; NSC-747856; AZD221; CHEBI:83766; 4-[[3-[4-(Cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorophenyl]methyl]-2H-phthalazin-1-one; MFCD13185161; AZ-2281; KEYLYNK-010 COMPONENT OLAPARIB; KU59436; KU 59436; 4-(3-{[4-(Cyclopropylcarbonyl)piperazin-1-Yl]carbonyl}-4-Fluorobenzyl)phthalazin-1(2h)-One; 4-[3-(4-Cyclopropanecarbonyl-piperazine-1-carbonyl)-4-fluoro-benzyl]-2H-phthalazin-1-one; NSC 747856; OLAPARIB COMPONENT OF KEYLYNK-010; OLAPARIB (MART.); OLAPARIB [MART.]; Olaparib (DISCONTINUED); Olaparib [INN]; (2H)-Phthalazinone, 4-((3-((4-(cyclopropylcarbonyl)-1-piperazinyl)carbonyl)-4-fluorophenyl)methyl)-; 1(2H)-Phthalazinone, 4-[[3-[[4-(cyclopropylcarbonyl)-1-piperazinyl]carbonyl]-4-fluorophenyl]methyl]-; 4-[(3-{[4-(Cyclopropylcarbonyl)piperazin-1-yl]carbonyl}-4-fluorophenyl)methyl]phthalazin-1(2H)-one; PIPERAZINE, 1-(CYCLOPROPYLCARBONYL)-4-(5-((3,4-DIHYDRO-4-OXO-1-PHTHALAZINYL)METHYL)-2-FLUOROBENZOYL)-; Olaparib (AZD2281; Ku-0059436); C24H23FN4O3; Olaparib (AZD2281); 4-(3-((4-(cyclopropylcarbonyl)piperazin-1-yl)carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; 4-[3-[4-(Cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorobenzyl]phthalazin-1(2H)-one; Olaparib- Bio-X; Lynparza (TN); 09L; 1(2H)-PHTHALAZINONE, 4-((3-((4-(CYCLOPROPYLCARBONYL)-1-PIPERAZINYL)CARBONYL)-4-FLUOROPHENYL)METHYL)-; 4-((3-((4-(CYCLOPROPYLCARBONYL)PIPERAZIN-1-YL)CARBONYL)-4-FLUOROPHENYL)METHYL)PHTHALAZIN-1(2H)-ONE; 4-((3-{(4-(cyclopropylcarbonyl)piperazin-1-yl)carbonyl}-4-fluorophenyl)methyl)phthalazin-1(2H)-one; 4-[[3-[[4-(Cyclopropylcarbonyl)-1-piperazinyl]carbonyl]-4-fluorophenyl]methyl]-1(2H)-phthalazinone; AZD 2281; KU 0059436; KU 59436; 1-(Cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazinyl)methyl]-2-fluorobenzoyl]piperazine;; AZD2281(olaparib); OLAPARIB [USAN]; OLAPARIB [JAN]; OLAPARIB [MI]; OLAPARIB [VANDF]; OLAPARIB [WHO-DD]; AZD-2281 (Olaparib); PARP inhibitor AZD2281; Olaparib (JAN/USAN/INN); 937799-91-2; MLS006010185; SCHEMBL426568; Olaparib, KU-0059436; OLAPARIB [ORANGE BOOK]; CHEMBL521686; GTPL7519; BDBM27566; DTXSID60917988; EX-A002; L01XX46; FDLYAMZZIXQODN-UHFFFAOYSA-N; BCPP000360; GLXC-02796; HMS3295I09; HMS3426C03; HMS3654G13; HMS3746K07; HMS3870H03; AMY10295; BCP01872; 763113-22-0, Lynparza,; NSC747856; NSC753686; s1060; AKOS005145764; AC-7939; BCP9000363; CCG-264799; CS-0075; DB09074; EX-7210; NSC-753686; SB14617; SS-4573; AZD2281,Olaparib, KU-0059436; NCGC00238451-01; NCGC00238451-02; NCGC00238451-08; NCGC00238451-09; NCGC00238451-11; 4-[(3-{[4-Cyclopropylcarbonyl)piperazin-4-yl]carbonyl}-4-fluorophenyl)methyl]phtalazin-1(2H)-one; 4-[[3-[[4-(Cyclopropylcarbonyl)-1-piperazinyl]carbonyl]-4-fluorophenyl]methyl]-1(2H)-phthalazinone; 4-{[3-(4-cyclopropanecarbonylpiperazine-1-carbonyl)-4-fluorophenyl]methyl}-1,2-dihydrophthalazin-1-one; BO164169; HY-10162; SMR004701291; SY040527; A9666; NS00072449; SW218142-2; D09730; EN300-7542225; J-503540; Q7083106; AZD2281 , KU0059436; BRD-K02113016-001-08-9; BRD-K02113016-001-09-7; Z2227698469; 1-(cyclopropylcarbonyl)-4-[5-[(3,4-dihydro-4-oxo-1-phthalazine; 4-(3-(1-(cyclopropanecarbonyl)piperazine-4-carbonyl)-4-fluorobenzyl)phthalazin-1(2H)-one; 1021843-02-6; 4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbonyl]-4-fluorophenyl}methyl)-1,2-dihydrophthalazin-1-one
|
| Chemical Identifiers |
- Formula
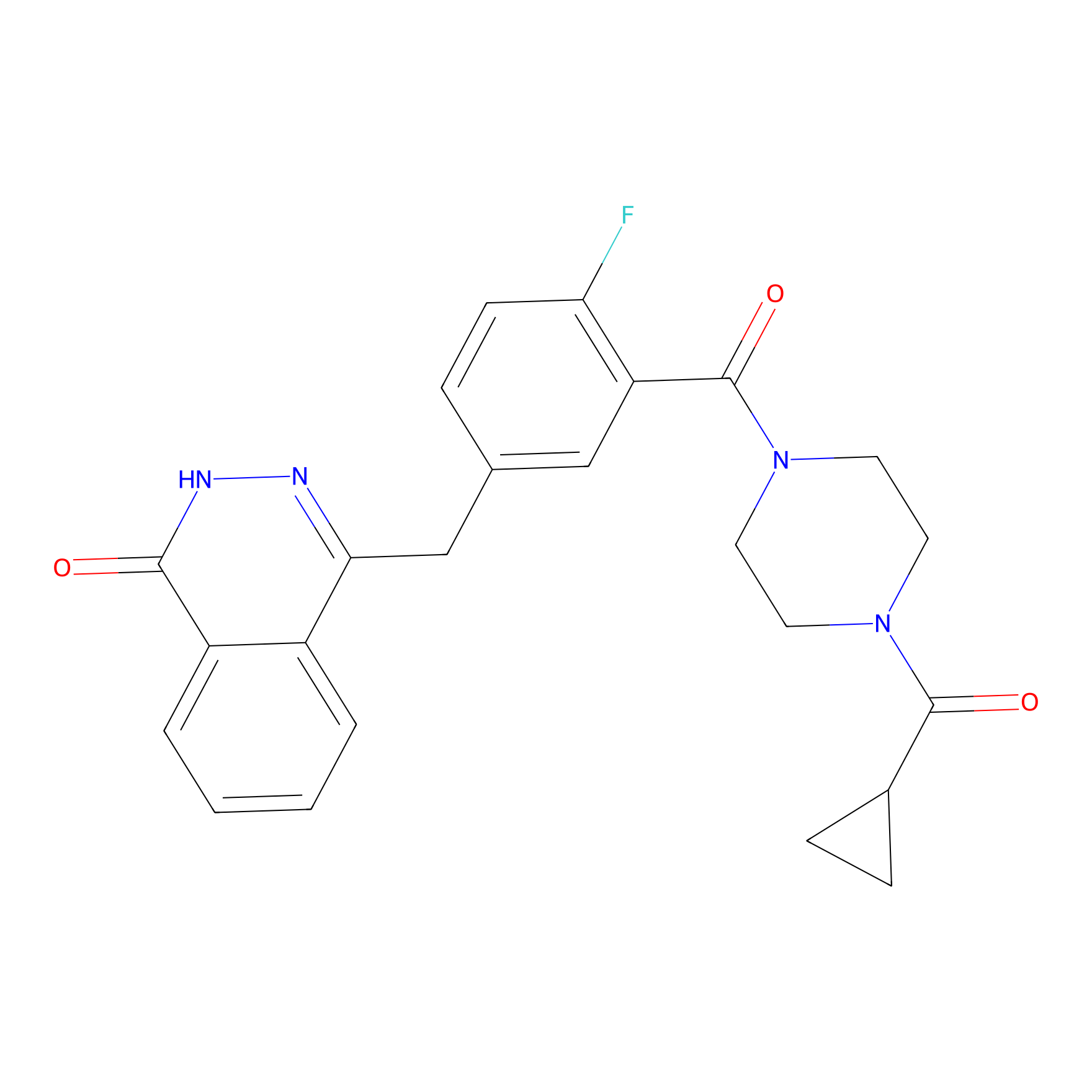
- C24H23FN4O3
- IUPAC Name
-
4-[[3-[4-(cyclopropanecarbonyl)piperazine-1-carbonyl]-4-fluorophenyl]methyl]-2H-phthalazin-1-one
- Canonical SMILES
-
C1CC1C(=O)N2CCN(CC2)C(=O)C3=C(C=CC(=C3)CC4=NNC(=O)C5=CC=CC=C54)F
- InChI
-
InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30)
- InChIKey
-
FDLYAMZZIXQODN-UHFFFAOYSA-N
|



 Download The Altas
Download The Altas