Details of the Target
General Information of Target
| Target ID | LDTP11576 | |||||
|---|---|---|---|---|---|---|
| Target Name | NACHT, LRR and PYD domains-containing protein 1 (NLRP1) | |||||
| Gene Name | NLRP1 | |||||
| Gene ID | 22861 | |||||
| Synonyms |
CARD7; DEFCAP; KIAA0926; NAC; NALP1; NACHT, LRR and PYD domains-containing protein 1; EC 3.4.-.-; EC 3.6.4.-; Caspase recruitment domain-containing protein 7; Death effector filament-forming ced-4-like apoptosis protein; Nucleotide-binding domain and caspase recruitment domain) [Cleaved into: NACHT, LRR and PYD domains-containing protein 1, C-terminus; NLRP1-CT; NACHT, LRR and PYD domains-containing protein 1, N-terminus; NLRP1-NT)]
|
|||||
| 3D Structure | ||||||
| Sequence |
MPTVEELYRNYGILADATEQVGQHKDAYQVILDGVKGGTKEKRLAAQFIPKFFKHFPELA
DSAINAQLDLCEDEDVSIRRQAIKELPQFATGENLPRVADILTQLLQTDDSAEFNLVNNA LLSIFKMDAKGTLGGLFSQILQGEDIVRERAIKFLSTKLKTLPDEVLTKEVEELILTESK KVLEDVTGEEFVLFMKILSGLKSLQTVSGRQQLVELVAEQADLEQTFNPSDPDCVDRLLQ CTRQAVPLFSKNVHSTRFVTYFCEQVLPNLGTLTTPVEGLDIQLEVLKLLAEMSSFCGDM EKLETNLRKLFDKLLEYMPLPPEEAENGENAGNEEPKLQFSYVECLLYSFHQLGRKLPDF LTAKLNAEKLKDFKIRLQYFARGLQVYIRQLRLALQGKTGEALKTEENKIKVVALKITNN INVLIKDLFHIPPSYKSTVTLSWKPVQKVEIGQKRASEDTTSGSPPKKSSAGPKRDARQI YNPPSGKYSSNLGNFNYEQRGAFRGSRGGRGWGTRGNRSRGRLY |
|||||
| Target Type |
Literature-reported
|
|||||
| Target Bioclass |
Enzyme
|
|||||
| Family |
NLRP family
|
|||||
| Subcellular location |
Nucleus; Cytoplasm, cytosol; Inflammasome
|
|||||
| Function |
Acts as the sensor component of the NLRP1 inflammasome, which mediates inflammasome activation in response to various pathogen-associated signals, leading to subsequent pyroptosis . Inflammasomes are supramolecular complexes that assemble in the cytosol in response to pathogens and other damage-associated signals and play critical roles in innate immunity and inflammation. Acts as a recognition receptor (PRR): recognizes specific pathogens and other damage-associated signals, such as cleavage by some human enteroviruses and rhinoviruses, double-stranded RNA, UV-B irradiation, or Val-boroPro inhibitor, and mediates the formation of the inflammasome polymeric complex composed of NLRP1, CASP1 and PYCARD/ASC . In response to pathogen-associated signals, the N-terminal part of NLRP1 is degraded by the proteasome, releasing the cleaved C-terminal part of the protein (NACHT, LRR and PYD domains-containing protein 1, C-terminus), which polymerizes and associates with PYCARD/ASC to initiate the formation of the inflammasome complex: the NLRP1 inflammasome recruits pro-caspase-1 (proCASP1) and promotes caspase-1 (CASP1) activation, which subsequently cleaves and activates inflammatory cytokines IL1B and IL18 and gasdermin-D (GSDMD), leading to pyroptosis. In the absence of GSDMD expression, the NLRP1 inflammasome is able to recruit and activate CASP8, leading to activation of gasdermin-E (GSDME). Activation of NLRP1 inflammasome is also required for HMGB1 secretion; the active cytokines and HMGB1 stimulate inflammatory responses. Binds ATP and shows ATPase activity. Plays an important role in antiviral immunity and inflammation in the human airway epithelium. Specifically recognizes a number of pathogen-associated signals: upon infection by human rhinoviruses 14 and 16 (HRV-14 and HRV-16), NLRP1 is cleaved and activated which triggers NLRP1-dependent inflammasome activation and IL18 secretion. Positive-strand RNA viruses, such as Semliki forest virus and long dsRNA activate the NLRP1 inflammasome, triggering IL1B release in a NLRP1-dependent fashion. Acts as a direct sensor for long dsRNA and thus RNA virus infection. May also be activated by muramyl dipeptide (MDP), a fragment of bacterial peptidoglycan, in a NOD2-dependent manner. The NLRP1 inflammasome is also activated in response to UV-B irradiation causing ribosome collisions: ribosome collisions cause phosphorylation and activation of NLRP1 in a MAP3K20-dependent manner, leading to pyroptosis.; [NACHT, LRR and PYD domains-containing protein 1]: Constitutes the precursor of the NLRP1 inflammasome, which mediates autoproteolytic processing within the FIIND domain to generate the N-terminal and C-terminal parts, which are associated non-covalently in absence of pathogens and other damage-associated signals.; [NACHT, LRR and PYD domains-containing protein 1, N-terminus]: Regulatory part that prevents formation of the NLRP1 inflammasome: in absence of pathogens and other damage-associated signals, interacts with the C-terminal part of NLRP1 (NACHT, LRR and PYD domains-containing protein 1, C-terminus), preventing activation of the NLRP1 inflammasome. In response to pathogen-associated signals, this part is ubiquitinated and degraded by the proteasome, releasing the cleaved C-terminal part of the protein, which polymerizes and forms the NLRP1 inflammasome.; [NACHT, LRR and PYD domains-containing protein 1, C-terminus]: Constitutes the active part of the NLRP1 inflammasome. In absence of pathogens and other damage-associated signals, interacts with the N-terminal part of NLRP1 (NACHT, LRR and PYD domains-containing protein 1, N-terminus), preventing activation of the NLRP1 inflammasome. In response to pathogen-associated signals, the N-terminal part of NLRP1 is degraded by the proteasome, releasing this form, which polymerizes and associates with PYCARD/ASC to form of the NLRP1 inflammasome complex: the NLRP1 inflammasome complex then directly recruits pro-caspase-1 (proCASP1) and promotes caspase-1 (CASP1) activation, leading to gasdermin-D (GSDMD) cleavage and subsequent pyroptosis.; [Isoform 2]: It is unclear whether is involved in inflammasome formation. It is not cleaved within the FIIND domain, does not assemble into specks, nor promote IL1B release. However, in an vitro cell-free system, it has been shown to be activated by MDP.
|
|||||
| TTD ID | ||||||
| Uniprot ID | ||||||
| DrugMap ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
| ChEMBL ID | ||||||
Target Site Mutations in Different Cell Lines
| Cell line | Mutation details | Probe for labeling this protein in this cell | |||
|---|---|---|---|---|---|
| A498 | SNV: p.Q1271E | . | |||
| AGS | Substitution: p.M1184A | . | |||
| C32 | SNV: p.E724K | . | |||
| DEL | Substitution: p.M1184A | . | |||
| FTC133 | SNV: p.R35M | . | |||
| HCC1143 | SNV: p.R890K | . | |||
| HCC1419 | Substitution: p.M1184A | . | |||
| HCT15 | SNV: p.R834L; p.G1074R | . | |||
| HG3 | Substitution: p.M1184A | DBIA Probe Info | |||
| HT | SNV: p.S821I | . | |||
| IGROV1 | SNV: p.K487N | . | |||
| IPC298 | SNV: p.L844S | . | |||
| Ishikawa (Heraklio) 02 ER | Deletion: p.E685DfsTer74 | . | |||
| MCC26 | SNV: p.L971M | . | |||
| MCF7 | SNV: p.P242Q | . | |||
| MFE319 | SNV: p.E23G; p.G1016Ter | . | |||
| MKN1 | SNV: p.V519L | . | |||
| MOLM13 | SNV: p.V937I | . | |||
| MOLT4 | SNV: p.R308Ter | . | |||
| NCIH716 | Substitution: p.M1184A | . | |||
| OVK18 | SNV: p.A880V | . | |||
| SW756 | SNV: p.L610V Substitution: p.M1184A |
DBIA Probe Info | |||
| TE4 | SNV: p.W580C | . | |||
| TOV21G | Deletion: p.K583RfsTer14 | . | |||
| WM115 | SNV: p.D570N | . | |||
| WM2664 | SNV: p.D570N | DBIA Probe Info | |||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
DBIA Probe Info |
 |
C1310(11.30); C264(89.22); C11(12.52) | LDD0209 | [1] | |
|
IA-alkyne Probe Info |
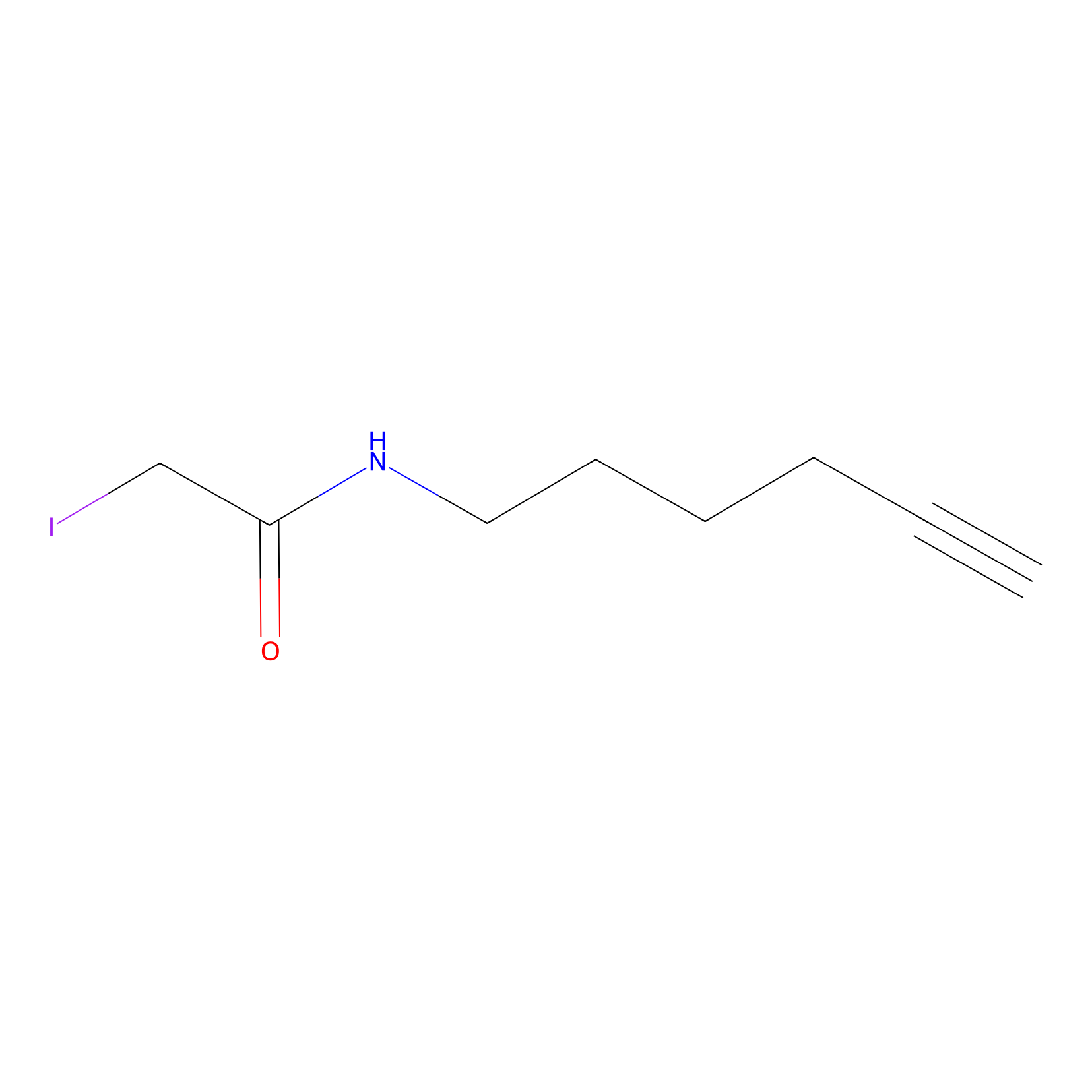 |
C264(6.10) | LDD1703 | [2] | |
|
IPM Probe Info |
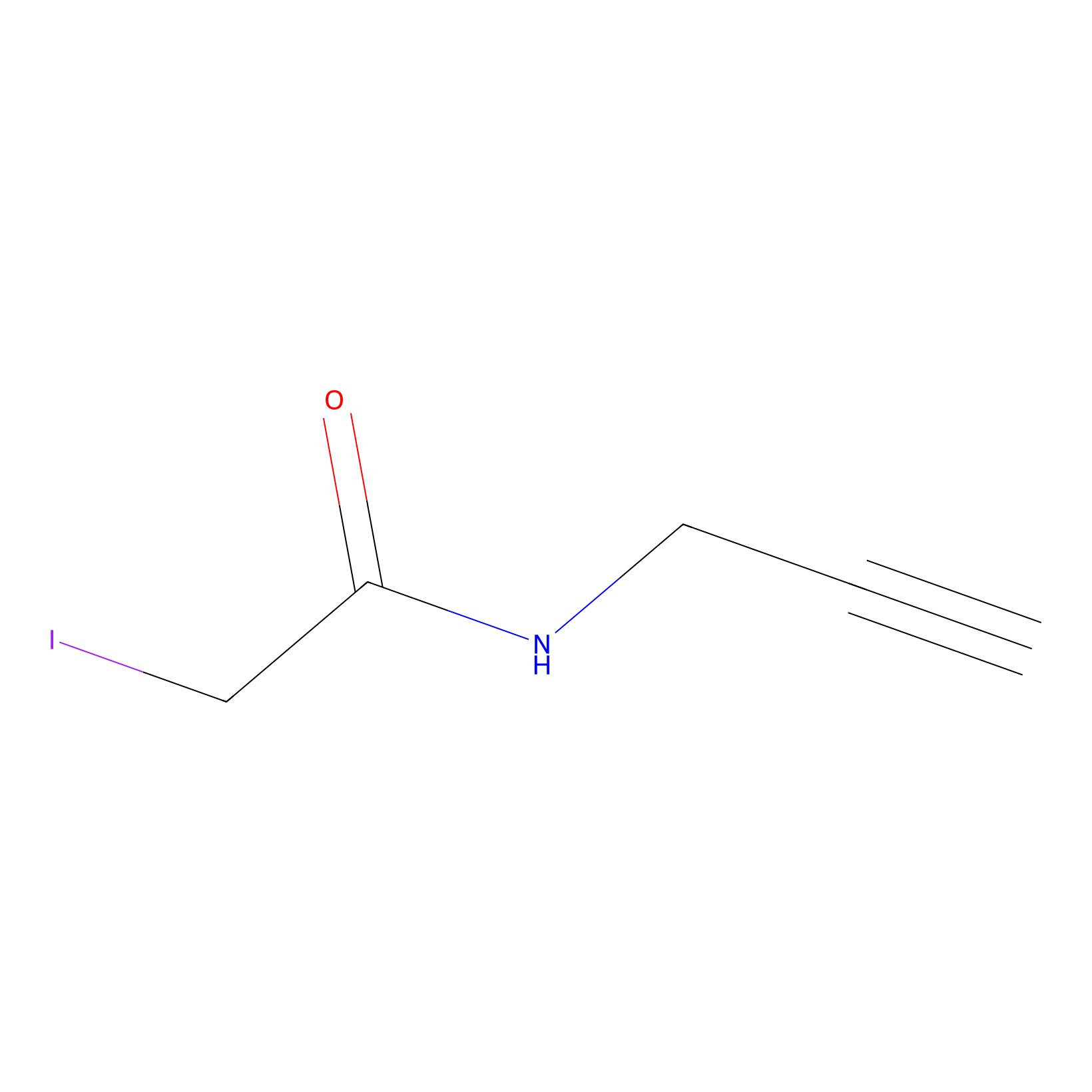 |
C837(2.73) | LDD1701 | [3] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0214 | AC1 | HEK-293T | C943(1.02) | LDD1507 | [4] |
| LDCM0215 | AC10 | HEK-293T | C943(1.04) | LDD1508 | [4] |
| LDCM0226 | AC11 | HEK-293T | C943(0.97) | LDD1509 | [4] |
| LDCM0237 | AC12 | HEK-293T | C943(0.98) | LDD1510 | [4] |
| LDCM0276 | AC17 | HEK-293T | C943(0.94) | LDD1515 | [4] |
| LDCM0277 | AC18 | HEK-293T | C943(0.98) | LDD1516 | [4] |
| LDCM0278 | AC19 | HEK-293T | C943(1.04) | LDD1517 | [4] |
| LDCM0279 | AC2 | HEK-293T | C943(1.00) | LDD1518 | [4] |
| LDCM0280 | AC20 | HEK-293T | C943(1.02) | LDD1519 | [4] |
| LDCM0284 | AC24 | HEK-293T | C943(0.97) | LDD1523 | [4] |
| LDCM0285 | AC25 | HEK-293T | C943(1.05) | LDD1524 | [4] |
| LDCM0286 | AC26 | HEK-293T | C943(1.02) | LDD1525 | [4] |
| LDCM0287 | AC27 | HEK-293T | C943(1.01) | LDD1526 | [4] |
| LDCM0288 | AC28 | HEK-293T | C943(1.02) | LDD1527 | [4] |
| LDCM0290 | AC3 | HEK-293T | C943(1.01) | LDD1529 | [4] |
| LDCM0293 | AC32 | HEK-293T | C943(1.03) | LDD1532 | [4] |
| LDCM0294 | AC33 | HEK-293T | C943(1.04) | LDD1533 | [4] |
| LDCM0295 | AC34 | HEK-293T | C943(1.01) | LDD1534 | [4] |
| LDCM0296 | AC35 | HEK-293T | C943(1.02) | LDD1535 | [4] |
| LDCM0297 | AC36 | HEK-293T | C943(1.00) | LDD1536 | [4] |
| LDCM0301 | AC4 | HEK-293T | C943(1.01) | LDD1540 | [4] |
| LDCM0302 | AC40 | HEK-293T | C943(0.96) | LDD1541 | [4] |
| LDCM0303 | AC41 | HEK-293T | C943(0.98) | LDD1542 | [4] |
| LDCM0304 | AC42 | HEK-293T | C943(1.07) | LDD1543 | [4] |
| LDCM0305 | AC43 | HEK-293T | C943(1.02) | LDD1544 | [4] |
| LDCM0306 | AC44 | HEK-293T | C943(1.04) | LDD1545 | [4] |
| LDCM0310 | AC48 | HEK-293T | C943(1.01) | LDD1549 | [4] |
| LDCM0311 | AC49 | HEK-293T | C943(0.94) | LDD1550 | [4] |
| LDCM0313 | AC50 | HEK-293T | C943(0.96) | LDD1552 | [4] |
| LDCM0314 | AC51 | HEK-293T | C943(0.99) | LDD1553 | [4] |
| LDCM0315 | AC52 | HEK-293T | C943(1.01) | LDD1554 | [4] |
| LDCM0319 | AC56 | HEK-293T | C943(1.00) | LDD1558 | [4] |
| LDCM0320 | AC57 | HEK-293T | C943(1.04) | LDD1559 | [4] |
| LDCM0321 | AC58 | HEK-293T | C943(1.04) | LDD1560 | [4] |
| LDCM0322 | AC59 | HEK-293T | C943(0.99) | LDD1561 | [4] |
| LDCM0324 | AC60 | HEK-293T | C943(1.02) | LDD1563 | [4] |
| LDCM0328 | AC64 | HEK-293T | C943(1.02) | LDD1567 | [4] |
| LDCM0345 | AC8 | HEK-293T | C943(1.08) | LDD1569 | [4] |
| LDCM0356 | AKOS034007680 | HEK-293T | C943(1.01) | LDD1570 | [4] |
| LDCM0275 | AKOS034007705 | HEK-293T | C943(1.04) | LDD1514 | [4] |
| LDCM0369 | CL100 | HEK-293T | C943(1.13) | LDD1573 | [4] |
| LDCM0373 | CL104 | HEK-293T | C943(1.02) | LDD1577 | [4] |
| LDCM0377 | CL108 | HEK-293T | C943(0.95) | LDD1581 | [4] |
| LDCM0382 | CL112 | HEK-293T | C943(0.85) | LDD1586 | [4] |
| LDCM0386 | CL116 | HEK-293T | C943(1.04) | LDD1590 | [4] |
| LDCM0390 | CL12 | HEK-293T | C943(1.03) | LDD1594 | [4] |
| LDCM0391 | CL120 | HEK-293T | C943(0.98) | LDD1595 | [4] |
| LDCM0395 | CL124 | HEK-293T | C943(1.03) | LDD1599 | [4] |
| LDCM0399 | CL128 | HEK-293T | C943(1.04) | LDD1603 | [4] |
| LDCM0403 | CL16 | HEK-293T | C943(0.92) | LDD1607 | [4] |
| LDCM0404 | CL17 | HEK-293T | C943(1.08) | LDD1608 | [4] |
| LDCM0405 | CL18 | HEK-293T | C943(0.96) | LDD1609 | [4] |
| LDCM0406 | CL19 | HEK-293T | C943(1.07) | LDD1610 | [4] |
| LDCM0408 | CL20 | HEK-293T | C943(1.06) | LDD1612 | [4] |
| LDCM0412 | CL24 | HEK-293T | C943(1.14) | LDD1616 | [4] |
| LDCM0416 | CL28 | HEK-293T | C943(1.03) | LDD1620 | [4] |
| LDCM0417 | CL29 | HEK-293T | C943(1.00) | LDD1621 | [4] |
| LDCM0419 | CL30 | HEK-293T | C943(0.94) | LDD1623 | [4] |
| LDCM0420 | CL31 | HEK-293T | C943(1.04) | LDD1624 | [4] |
| LDCM0421 | CL32 | HEK-293T | C943(1.00) | LDD1625 | [4] |
| LDCM0425 | CL36 | HEK-293T | C943(1.17) | LDD1629 | [4] |
| LDCM0429 | CL4 | HEK-293T | C943(1.04) | LDD1633 | [4] |
| LDCM0430 | CL40 | HEK-293T | C943(0.95) | LDD1634 | [4] |
| LDCM0431 | CL41 | HEK-293T | C943(1.01) | LDD1635 | [4] |
| LDCM0432 | CL42 | HEK-293T | C943(1.01) | LDD1636 | [4] |
| LDCM0433 | CL43 | HEK-293T | C943(1.02) | LDD1637 | [4] |
| LDCM0434 | CL44 | HEK-293T | C943(1.00) | LDD1638 | [4] |
| LDCM0438 | CL48 | HEK-293T | C943(1.16) | LDD1642 | [4] |
| LDCM0440 | CL5 | HEK-293T | C943(1.02) | LDD1644 | [4] |
| LDCM0443 | CL52 | HEK-293T | C943(0.99) | LDD1646 | [4] |
| LDCM0444 | CL53 | HEK-293T | C943(1.25) | LDD1647 | [4] |
| LDCM0445 | CL54 | HEK-293T | C943(1.26) | LDD1648 | [4] |
| LDCM0446 | CL55 | HEK-293T | C943(1.11) | LDD1649 | [4] |
| LDCM0447 | CL56 | HEK-293T | C943(1.12) | LDD1650 | [4] |
| LDCM0451 | CL6 | HEK-293T | C943(1.03) | LDD1654 | [4] |
| LDCM0452 | CL60 | HEK-293T | C943(1.29) | LDD1655 | [4] |
| LDCM0456 | CL64 | HEK-293T | C943(1.01) | LDD1659 | [4] |
| LDCM0457 | CL65 | HEK-293T | C943(1.07) | LDD1660 | [4] |
| LDCM0458 | CL66 | HEK-293T | C943(1.05) | LDD1661 | [4] |
| LDCM0459 | CL67 | HEK-293T | C943(1.04) | LDD1662 | [4] |
| LDCM0460 | CL68 | HEK-293T | C943(1.10) | LDD1663 | [4] |
| LDCM0462 | CL7 | HEK-293T | C943(1.00) | LDD1665 | [4] |
| LDCM0465 | CL72 | HEK-293T | C943(1.07) | LDD1668 | [4] |
| LDCM0469 | CL76 | HEK-293T | C943(0.97) | LDD1672 | [4] |
| LDCM0470 | CL77 | HEK-293T | C943(1.07) | LDD1673 | [4] |
| LDCM0471 | CL78 | HEK-293T | C943(1.06) | LDD1674 | [4] |
| LDCM0472 | CL79 | HEK-293T | C943(1.01) | LDD1675 | [4] |
| LDCM0473 | CL8 | HEK-293T | C943(1.73) | LDD1676 | [4] |
| LDCM0474 | CL80 | HEK-293T | C943(1.11) | LDD1677 | [4] |
| LDCM0478 | CL84 | HEK-293T | C943(1.13) | LDD1681 | [4] |
| LDCM0482 | CL88 | HEK-293T | C943(1.12) | LDD1685 | [4] |
| LDCM0483 | CL89 | HEK-293T | C943(1.03) | LDD1686 | [4] |
| LDCM0485 | CL90 | HEK-293T | C943(1.24) | LDD1688 | [4] |
| LDCM0486 | CL91 | HEK-293T | C943(1.01) | LDD1689 | [4] |
| LDCM0487 | CL92 | HEK-293T | C943(1.05) | LDD1690 | [4] |
| LDCM0491 | CL96 | HEK-293T | C943(1.30) | LDD1694 | [4] |
| LDCM0022 | KB02 | T cell | C264(6.10) | LDD1703 | [2] |
| LDCM0023 | KB03 | Jurkat | C1310(11.30); C264(89.22); C11(12.52) | LDD0209 | [1] |
| LDCM0024 | KB05 | HMCB | C11(2.17) | LDD3312 | [5] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Enzyme
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Caspase-1 (CASP1) | Peptidase C14A family | P29466 | |||
Transporter and channel
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Apoptosis regulator Bcl-2 (BCL2) | Bcl-2 family | P10415 | |||
| Bcl-2-like protein 1 (BCL2L1) | Bcl-2 family | Q07817 | |||
Other
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Apoptosis-associated speck-like protein containing a CARD (PYCARD) | . | Q9ULZ3 | |||
References
