Details of the Target
General Information of Target
| Target ID | LDTP09216 | |||||
|---|---|---|---|---|---|---|
| Target Name | Serine palmitoyltransferase small subunit B (SPTSSB) | |||||
| Gene Name | SPTSSB | |||||
| Gene ID | 165679 | |||||
| Synonyms |
ADMP; C3orf57; SSSPTB; Serine palmitoyltransferase small subunit B; Protein ADMP; Small subunit of serine palmitoyltransferase B; ssSPTb |
|||||
| 3D Structure | ||||||
| Sequence |
MDLRRVKEYFSWLYYQYQIISCCAVLEPWERSMFNTILLTIIAMVVYTAYVFIPIHIRLA
WEFFSKICGYHSTISN |
|||||
| Target Bioclass |
Enzyme
|
|||||
| Family |
SPTSS family, SPTSSB subfamily
|
|||||
| Subcellular location |
Endoplasmic reticulum membrane
|
|||||
| Function |
Component of the serine palmitoyltransferase multisubunit enzyme (SPT) that catalyzes the initial and rate-limiting step in sphingolipid biosynthesis by condensing L-serine and activated acyl-CoA (most commonly palmitoyl-CoA) to form long-chain bases. The SPT complex is composed of SPTLC1, SPTLC2 or SPTLC3 and SPTSSA or SPTSSB. Within this complex, the heterodimer consisting of SPTLC1 and SPTLC2/SPTLC3 forms the catalytic core. Within the SPT complex, SPTSSB stimulates the catalytic activity and plays a role in substrate specificity. SPT complexes with this subunit showing a preference for longer acyl-CoAs. The SPTLC1-SPTLC2-SPTSSB complex shows a strong preference for C18-CoA substrate, while the SPTLC1-SPTLC3-SPTSSB isozyme displays an ability to use a broader range of acyl-CoAs, without apparent preference.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
Curcusone 37 Probe Info |
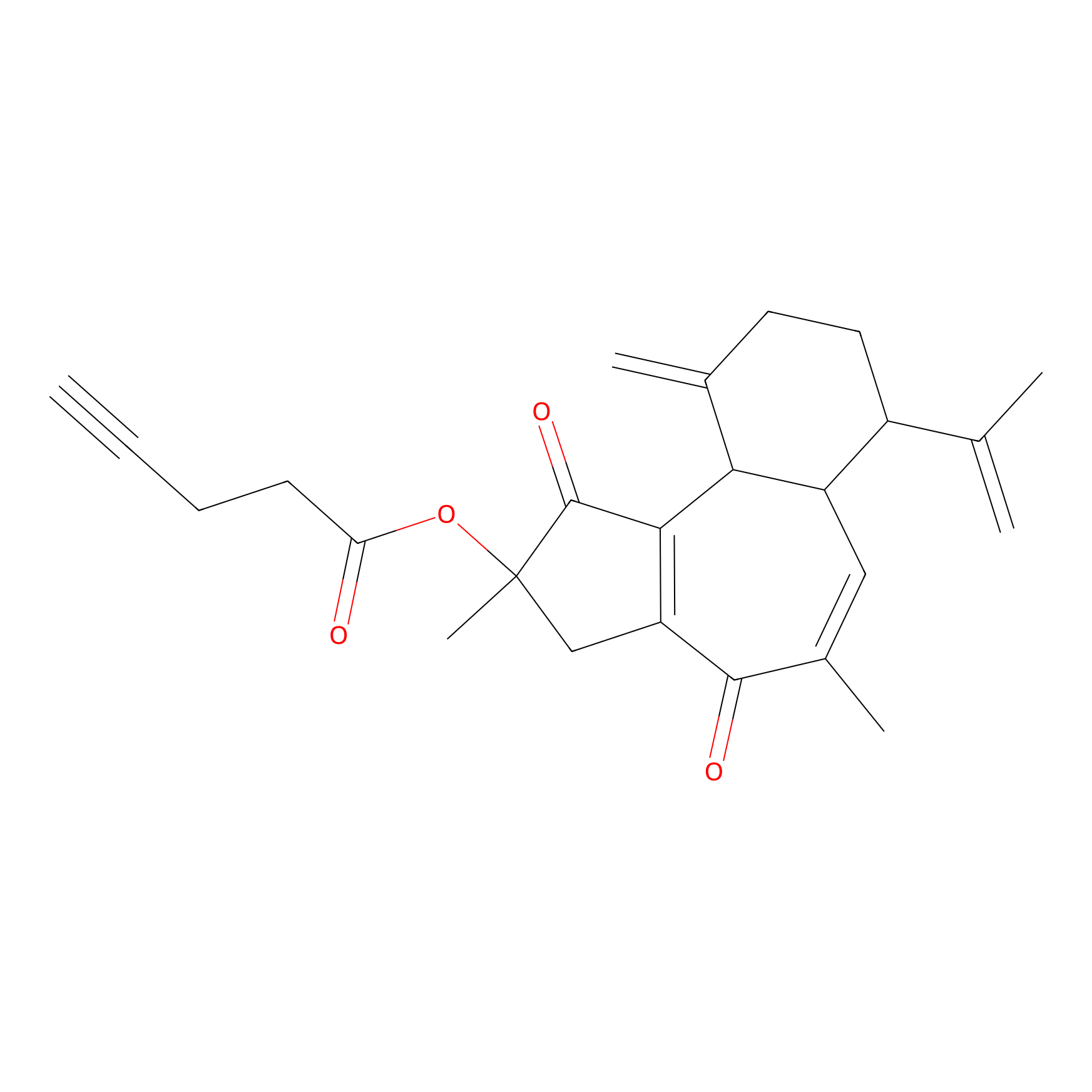 |
3.86 | LDD0188 | [1] | |
|
IPM Probe Info |
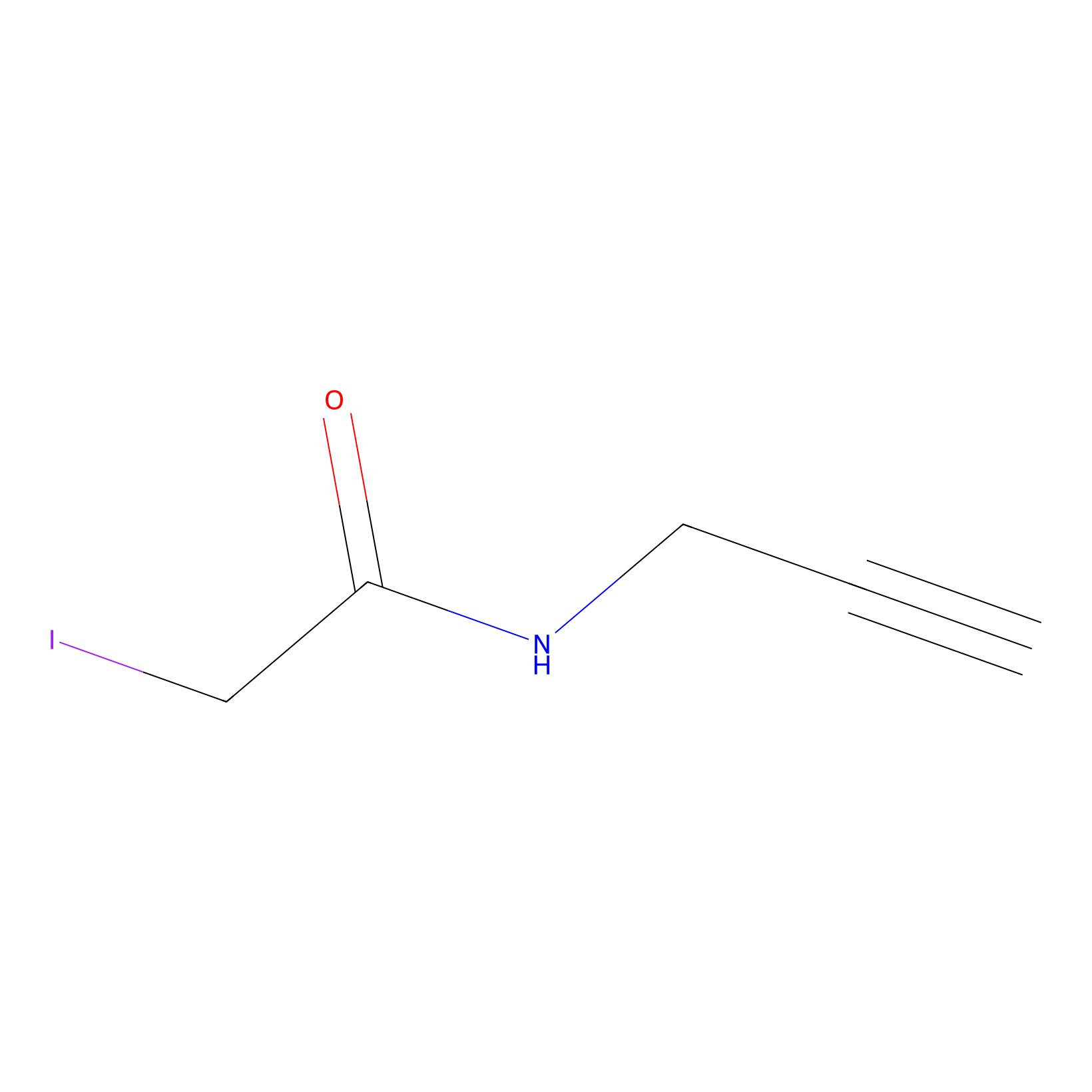 |
C68(2.11) | LDD0379 | [2] | |
|
NAIA_5 Probe Info |
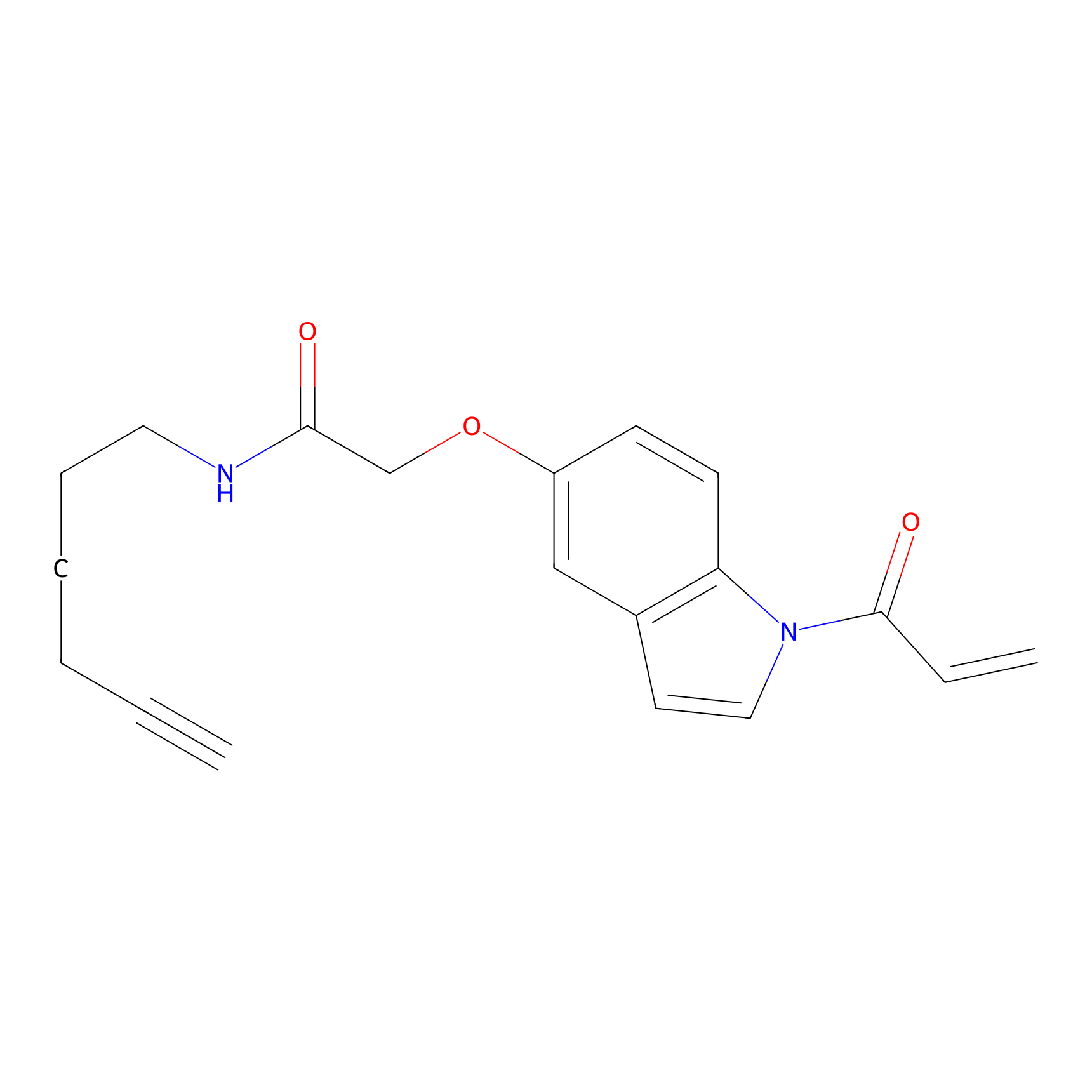 |
N.A. | LDD2224 | [3] | |
Competitor(s) Related to This Target
References
