Details of the Target
General Information of Target
| Target ID | LDTP05005 | |||||
|---|---|---|---|---|---|---|
| Target Name | Histone H2B type 1-C/E/F/G/I (H2BC4; H2BC6; H2BC7; H2BC8; H2BC10) | |||||
| Gene Name | H2BC4; H2BC6; H2BC7; H2BC8; H2BC10 | |||||
| Gene ID | 3017 | |||||
| Synonyms |
H2BFL; HIST1H2BC; H2BFH; HIST1H2BE; H2BFG; HIST1H2BF; H2BFA; HIST1H2BG; H2BFK; HIST1H2BI; Histone H2B type 1-C/E/F/G/I; Histone H2B.1 A; Histone H2B.a; H2B/a; Histone H2B.g; H2B/g; Histone H2B.h; H2B/h; Histone H2B.k; H2B/k; Histone H2B.l; H2B/l
|
|||||
| 3D Structure | ||||||
| Sequence |
MPEPAKSAPAPKKGSKKAVTKAQKKDGKKRKRSRKESYSVYVYKVLKQVHPDTGISSKAM
GIMNSFVNDIFERIAGEASRLAHYNKRSTITSREIQTAVRLLLPGELAKHAVSEGTKAVT KYTSSK |
|||||
| Target Bioclass |
Other
|
|||||
| Family |
Histone H2B family
|
|||||
| Subcellular location |
Nucleus
|
|||||
| Function |
Core component of nucleosome. Nucleosomes wrap and compact DNA into chromatin, limiting DNA accessibility to the cellular machineries which require DNA as a template. Histones thereby play a central role in transcription regulation, DNA repair, DNA replication and chromosomal stability. DNA accessibility is regulated via a complex set of post-translational modifications of histones, also called histone code, and nucleosome remodeling.; Has broad antibacterial activity. May contribute to the formation of the functional antimicrobial barrier of the colonic epithelium, and to the bactericidal activity of amniotic fluid.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
DBIA Probe Info |
 |
C73(1.50) | LDD2245 | [1] | |
|
HHS-475 Probe Info |
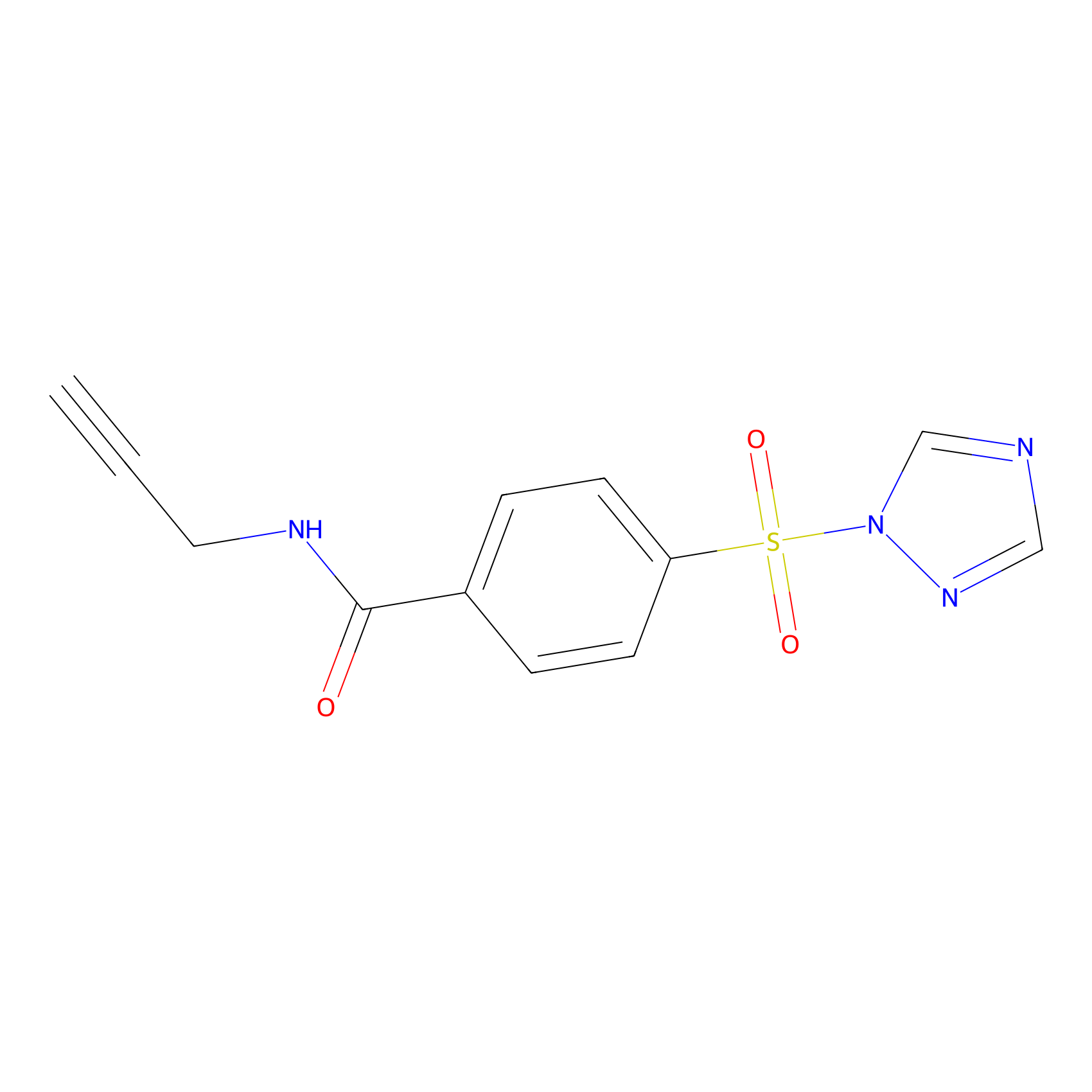 |
Y84(1.13) | LDD0264 | [2] | |
|
HHS-465 Probe Info |
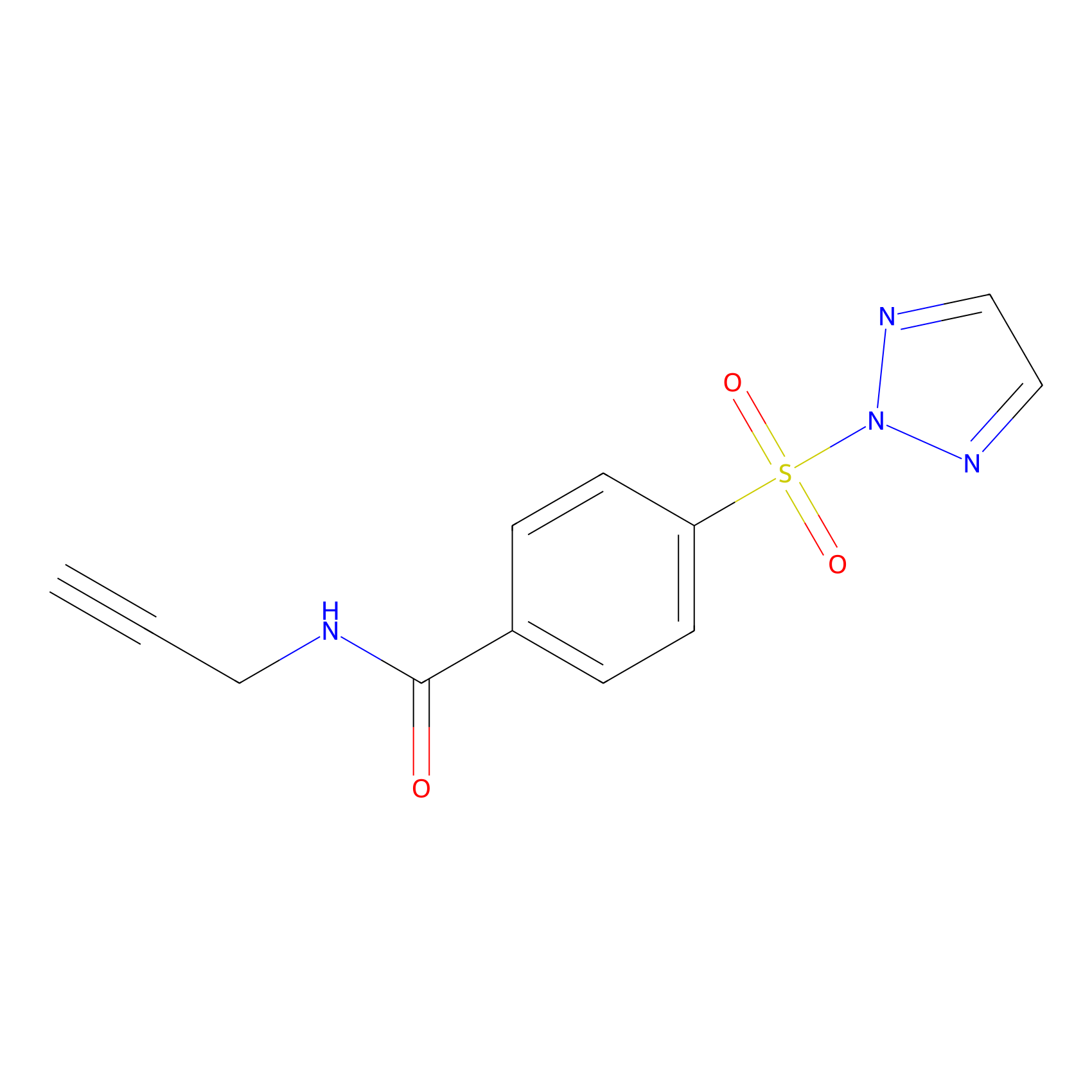 |
Y84(2.95) | LDD2237 | [3] | |
|
W1 Probe Info |
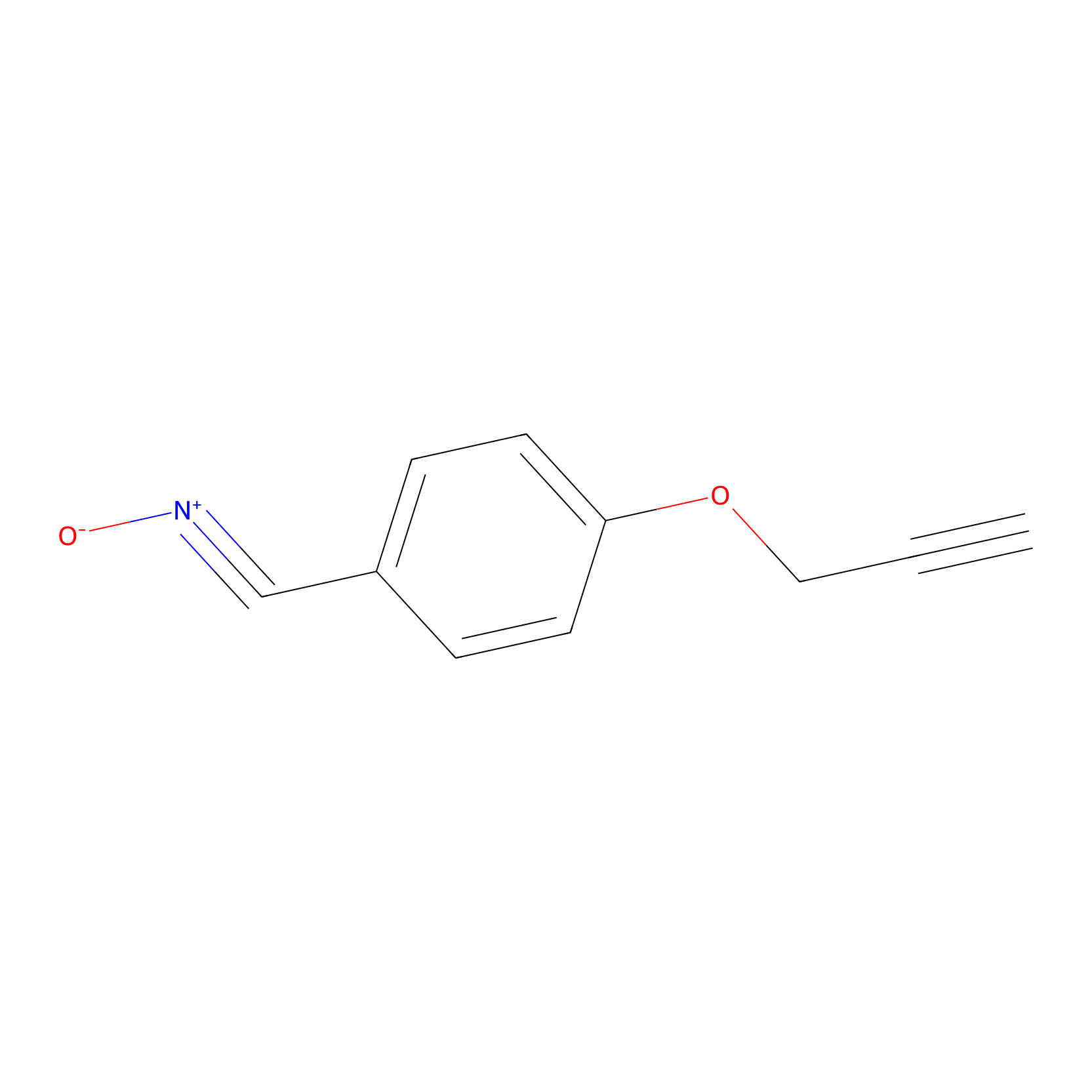 |
K86(0.59); K47(0.92); K109(0.99); K58(1.16) | LDD0237 | [4] | |
|
AMP probe Probe Info |
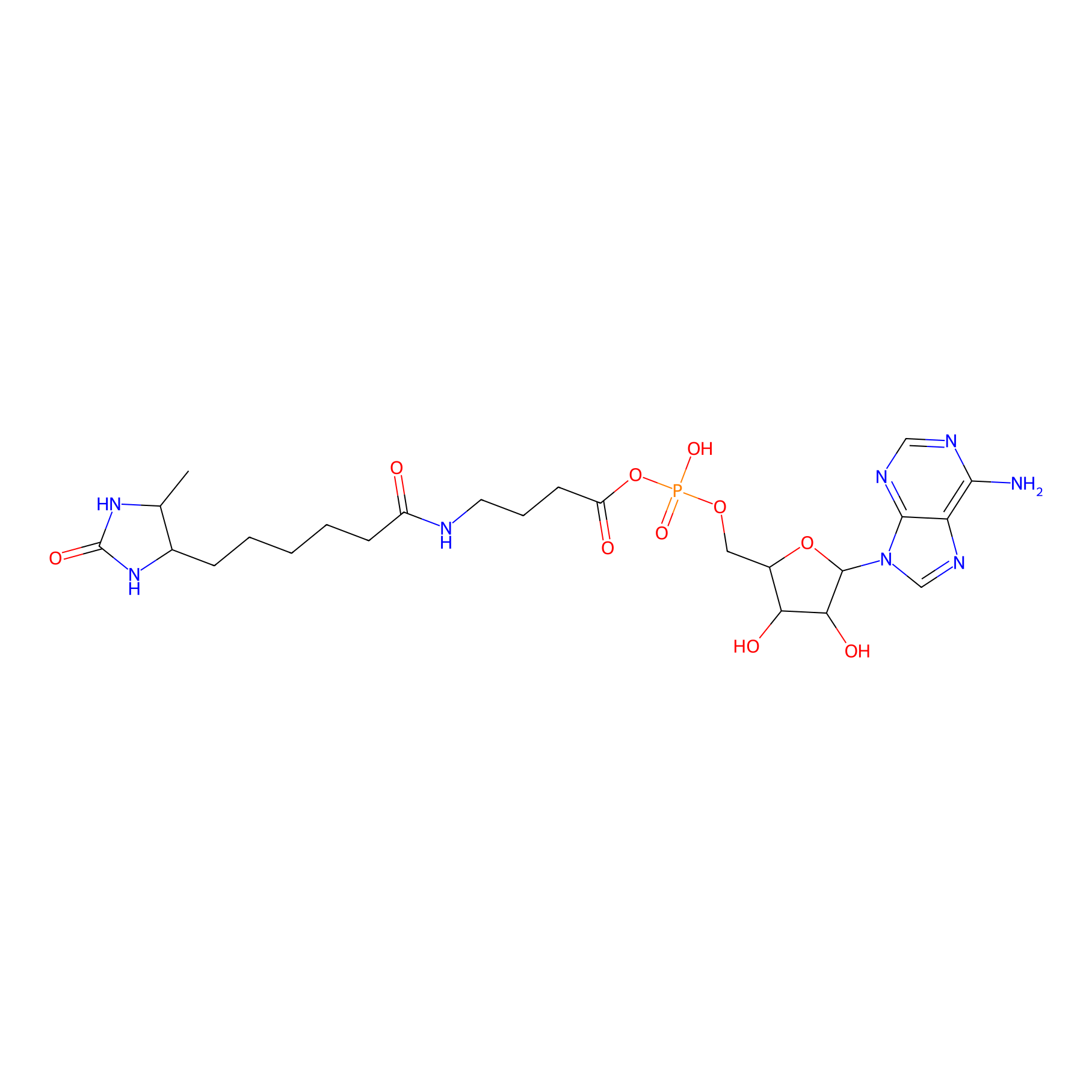 |
N.A. | LDD0200 | [5] | |
|
ATP probe Probe Info |
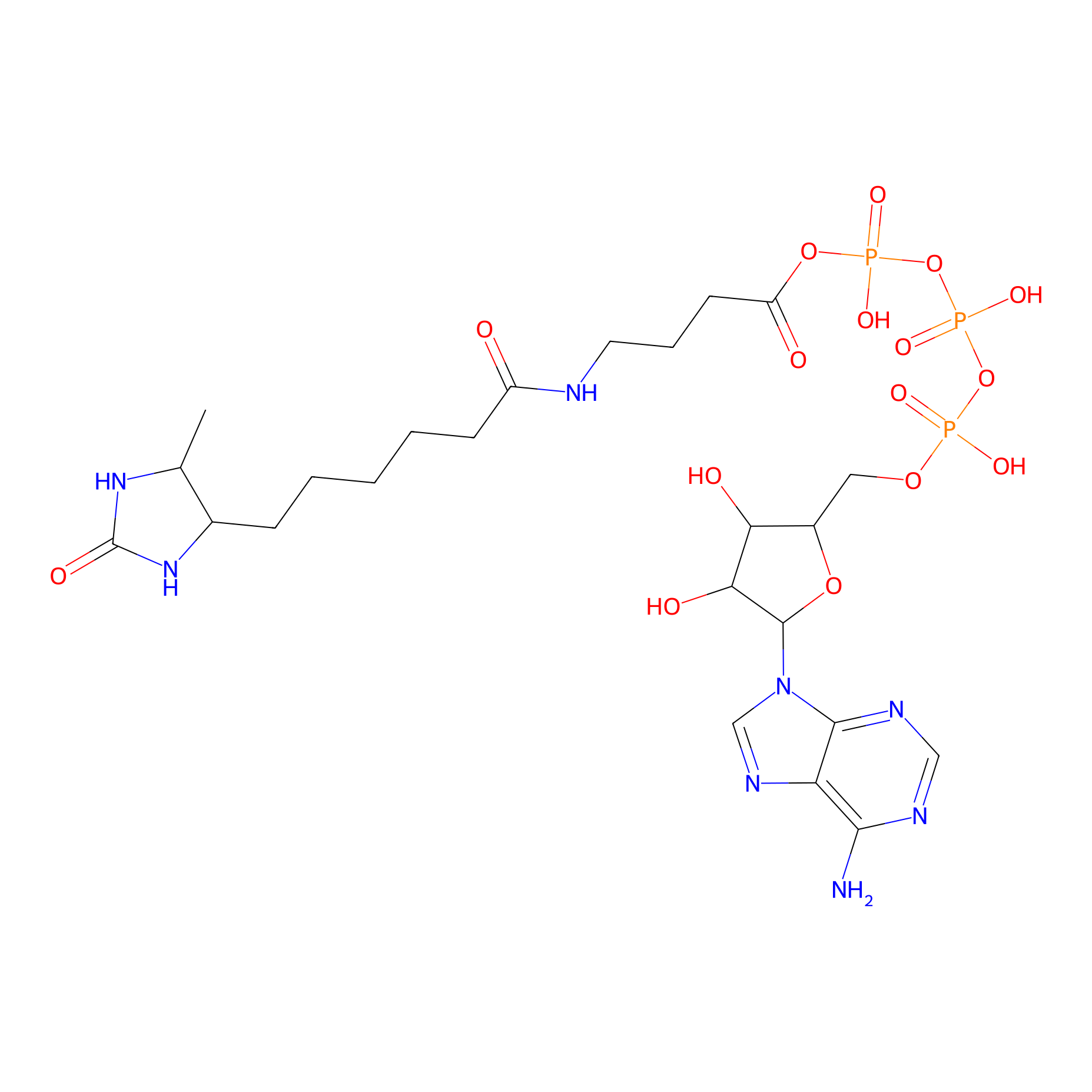 |
K47(0.00); K109(0.00); K117(0.00); K35(0.00) | LDD0199 | [5] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0116 | HHS-0101 | DM93 | Y84(1.13) | LDD0264 | [2] |
| LDCM0117 | HHS-0201 | DM93 | Y84(1.00) | LDD0265 | [2] |
| LDCM0118 | HHS-0301 | DM93 | Y84(1.27) | LDD0266 | [2] |
| LDCM0119 | HHS-0401 | DM93 | Y84(1.26) | LDD0267 | [2] |
| LDCM0120 | HHS-0701 | DM93 | Y84(1.74) | LDD0268 | [2] |
| LDCM0022 | KB02 | 697 | C73(1.50) | LDD2245 | [1] |
| LDCM0023 | KB03 | AN3-CA | C43(1.55) | LDD2681 | [1] |
| LDCM0024 | KB05 | AN3-CA | C43(1.76) | LDD3098 | [1] |
| LDCM0110 | W12 | Hep-G2 | K86(0.59); K47(0.92); K109(0.99); K58(1.16) | LDD0237 | [4] |
| LDCM0111 | W14 | Hep-G2 | E114(0.59); K47(0.95); K58(1.16); K109(1.27) | LDD0238 | [4] |
| LDCM0113 | W17 | Hep-G2 | K35(0.68); K109(1.25); K47(1.25); K58(1.35) | LDD0240 | [4] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Transporter and channel
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| AP-2 complex subunit mu (AP2M1) | Adaptor complexes medium subunit family | Q96CW1 | |||
Other
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Histone H2B type 1-C/E/F/G/I (H2BC4; H2BC6; H2BC7; H2BC8; H2BC10) | Histone H2B family | P62807 | |||
| TP53-binding protein 1 (TP53BP1) | . | Q12888 | |||
References
