Details of the Target
General Information of Target
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
BTD Probe Info |
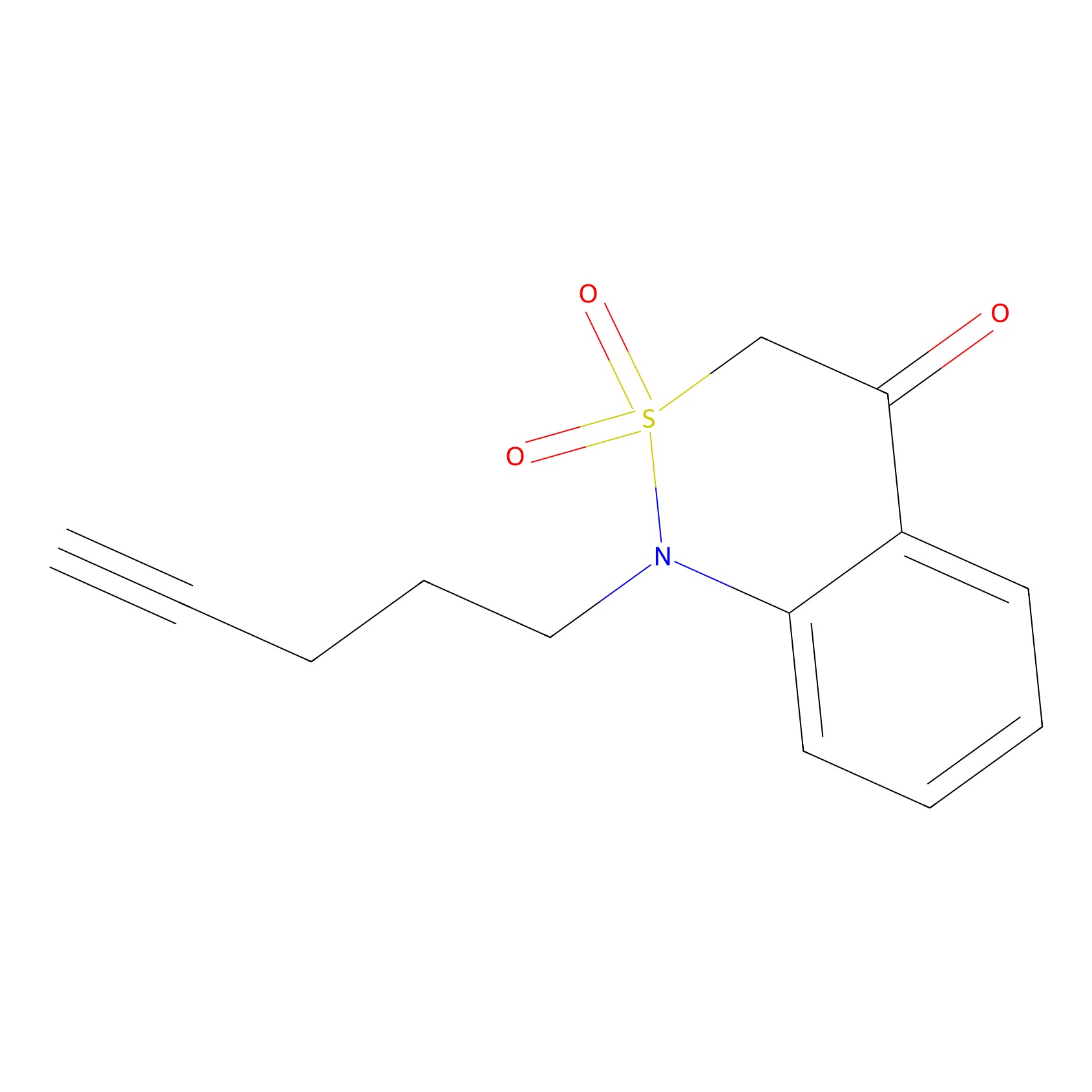 |
C281(1.18) | LDD2090 | [1] | |
|
IPM Probe Info |
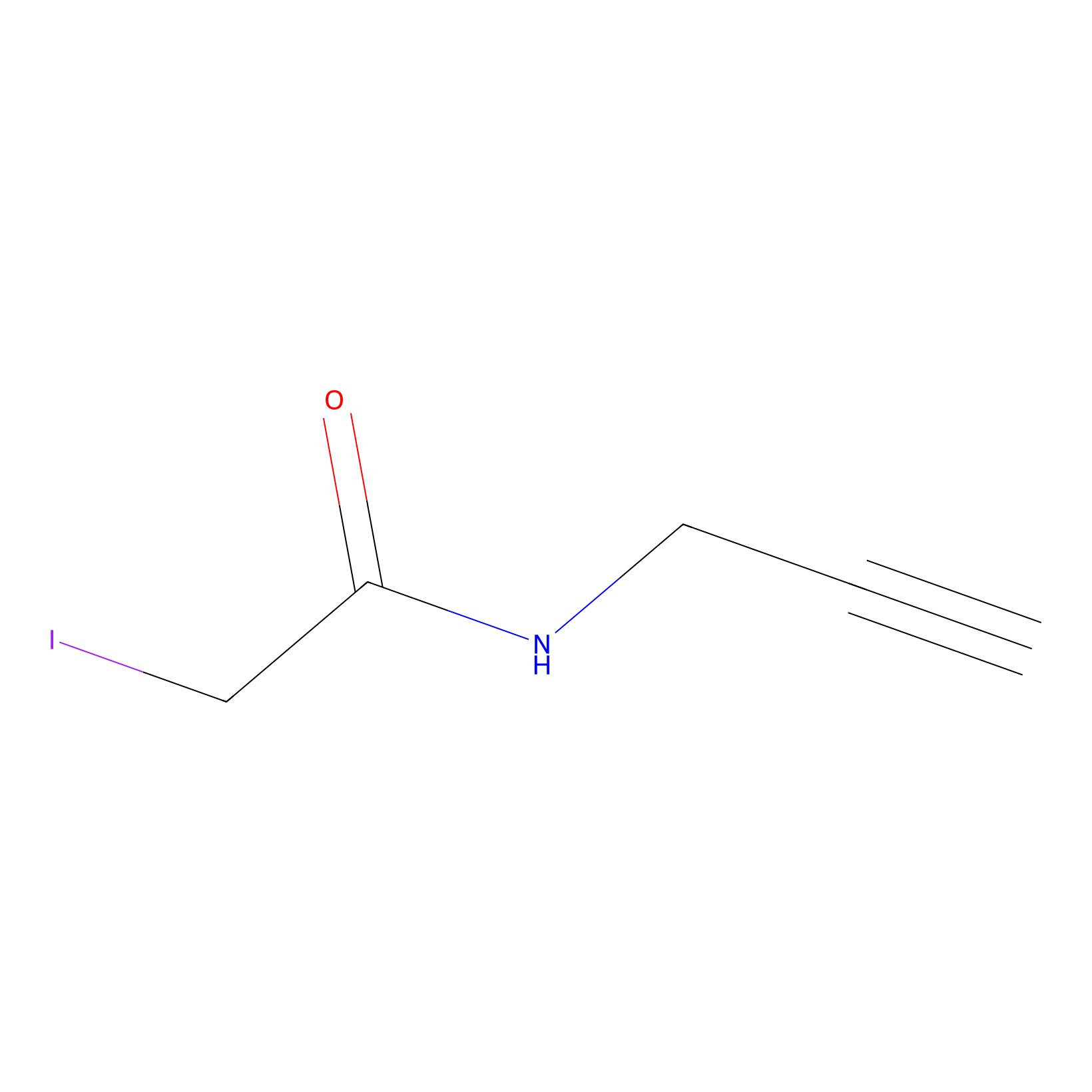 |
C233(2.62) | LDD1701 | [1] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0519 | 1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)-2-nitroethan-1-one | MDA-MB-231 | C281(0.52) | LDD2112 | [1] |
| LDCM0510 | 3-(4-(Hydroxydiphenylmethyl)piperidin-1-yl)-3-oxopropanenitrile | MDA-MB-231 | C281(2.10) | LDD2103 | [1] |
| LDCM0520 | AKOS000195272 | MDA-MB-231 | C281(1.33) | LDD2113 | [1] |
| LDCM0213 | Electrophilic fragment 2 | MDA-MB-231 | C233(1.35) | LDD1702 | [1] |
| LDCM0023 | KB03 | MDA-MB-231 | C233(2.62) | LDD1701 | [1] |
| LDCM0509 | N-(4-bromo-3,5-dimethylphenyl)-2-nitroacetamide | MDA-MB-231 | C281(1.82) | LDD2102 | [1] |
| LDCM0497 | Nucleophilic fragment 11b | MDA-MB-231 | C281(1.18) | LDD2090 | [1] |
| LDCM0499 | Nucleophilic fragment 12b | MDA-MB-231 | C281(1.73); C284(1.17) | LDD2092 | [1] |
| LDCM0501 | Nucleophilic fragment 13b | MDA-MB-231 | C281(2.61) | LDD2094 | [1] |
| LDCM0504 | Nucleophilic fragment 15a | MDA-MB-231 | C281(1.36) | LDD2097 | [1] |
| LDCM0505 | Nucleophilic fragment 15b | MDA-MB-231 | C281(1.10); C284(0.92) | LDD2098 | [1] |
| LDCM0515 | Nucleophilic fragment 20b | MDA-MB-231 | C284(0.58) | LDD2108 | [1] |
| LDCM0521 | Nucleophilic fragment 23b | MDA-MB-231 | C281(0.53) | LDD2114 | [1] |
| LDCM0522 | Nucleophilic fragment 24a | MDA-MB-231 | C281(1.06) | LDD2115 | [1] |
| LDCM0523 | Nucleophilic fragment 24b | MDA-MB-231 | C281(0.27) | LDD2116 | [1] |
| LDCM0525 | Nucleophilic fragment 25b | MDA-MB-231 | C281(0.51) | LDD2118 | [1] |
| LDCM0531 | Nucleophilic fragment 28b | MDA-MB-231 | C281(0.13) | LDD2124 | [1] |
| LDCM0554 | Nucleophilic fragment 7a | MDA-MB-231 | C281(0.46) | LDD2148 | [1] |
| LDCM0555 | Nucleophilic fragment 7b | MDA-MB-231 | C281(0.15); C284(2.83) | LDD2149 | [1] |
| LDCM0559 | Nucleophilic fragment 9b | MDA-MB-231 | C281(3.55) | LDD2153 | [1] |
