Details of the Target
General Information of Target
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
STPyne Probe Info |
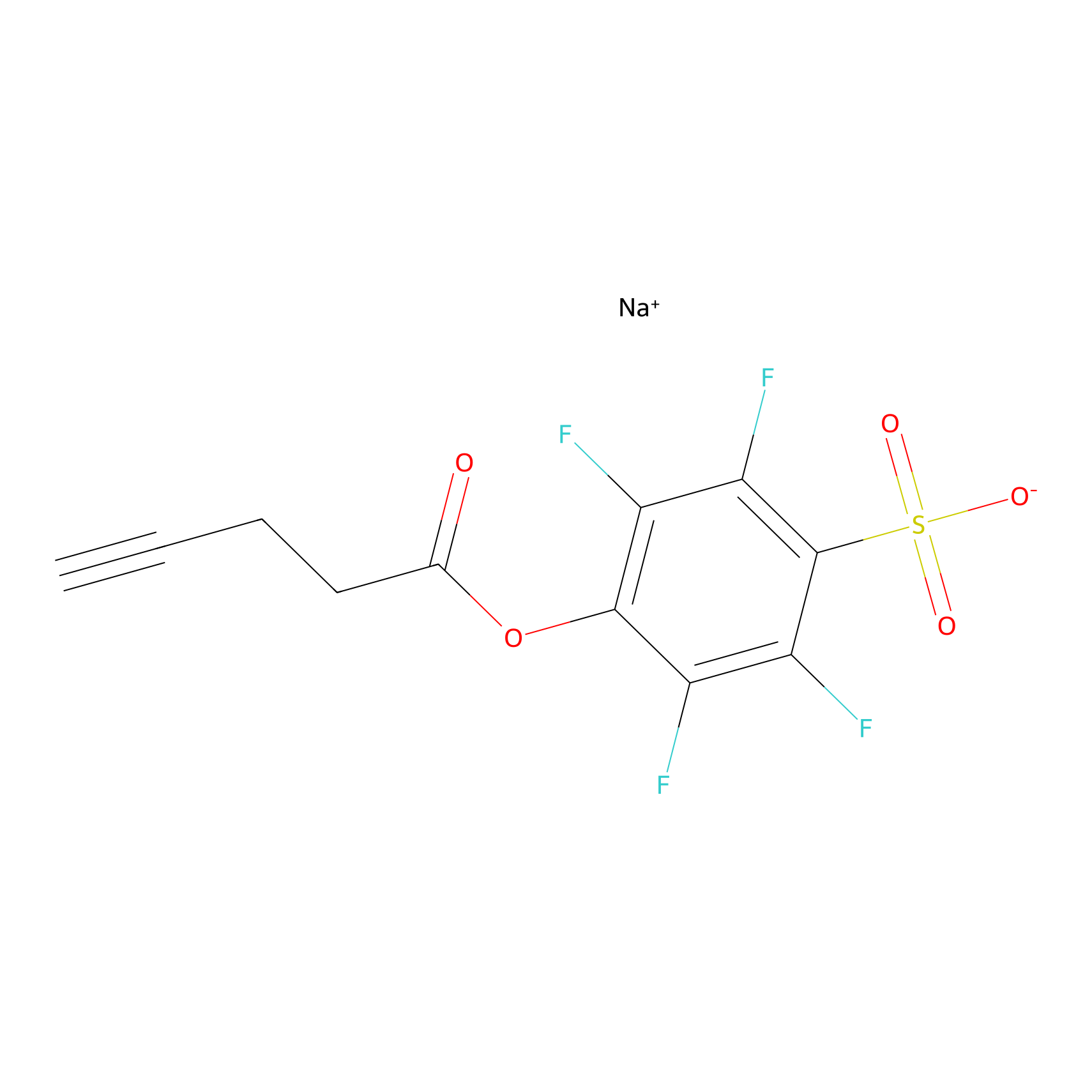 |
K131(12.55) | LDD2218 | [1] | |
|
DBIA Probe Info |
 |
C88(2.44); C134(3.09) | LDD3339 | [2] | |
|
IA-alkyne Probe Info |
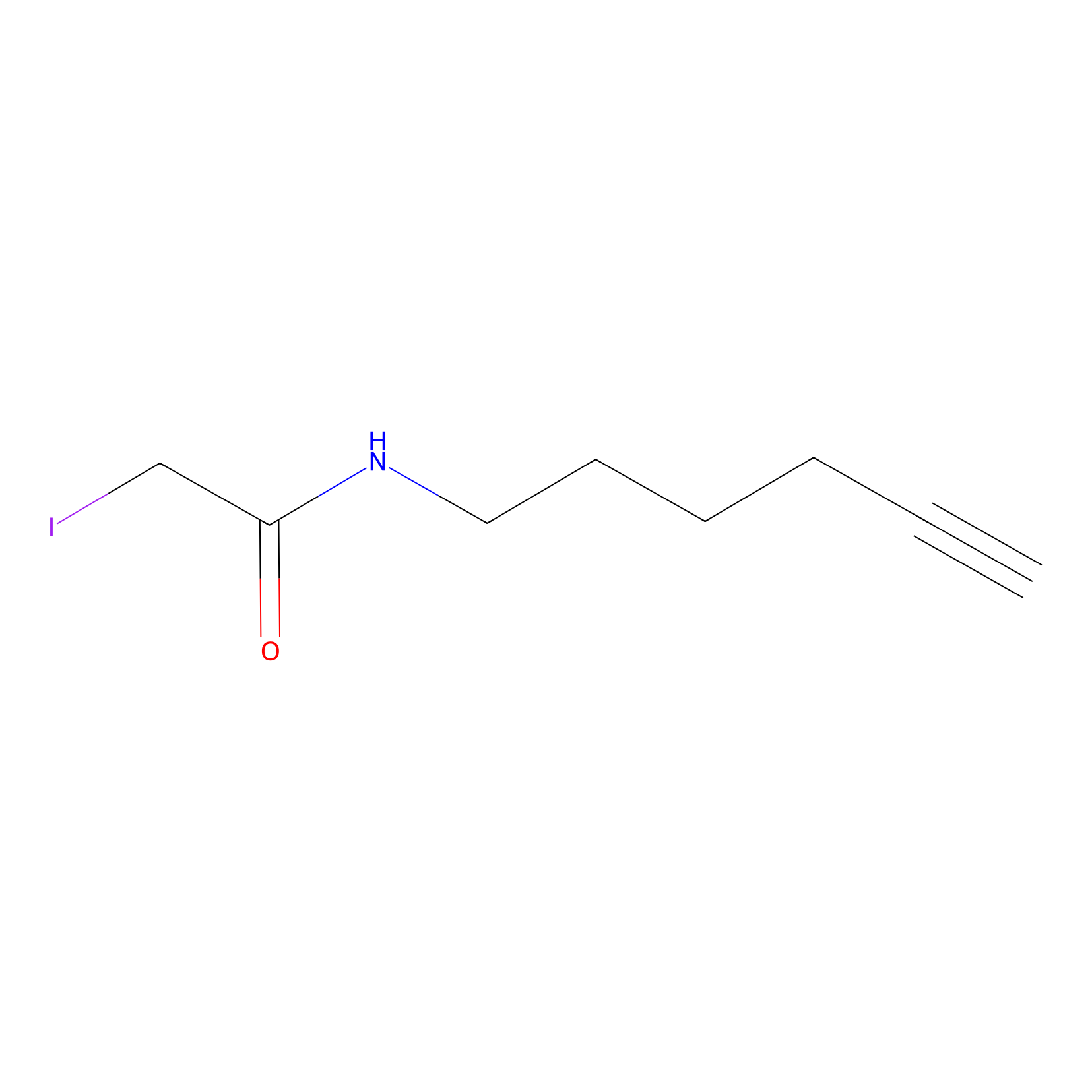 |
6.79 | LDD0433 | [3] | |
|
NAIA_5 Probe Info |
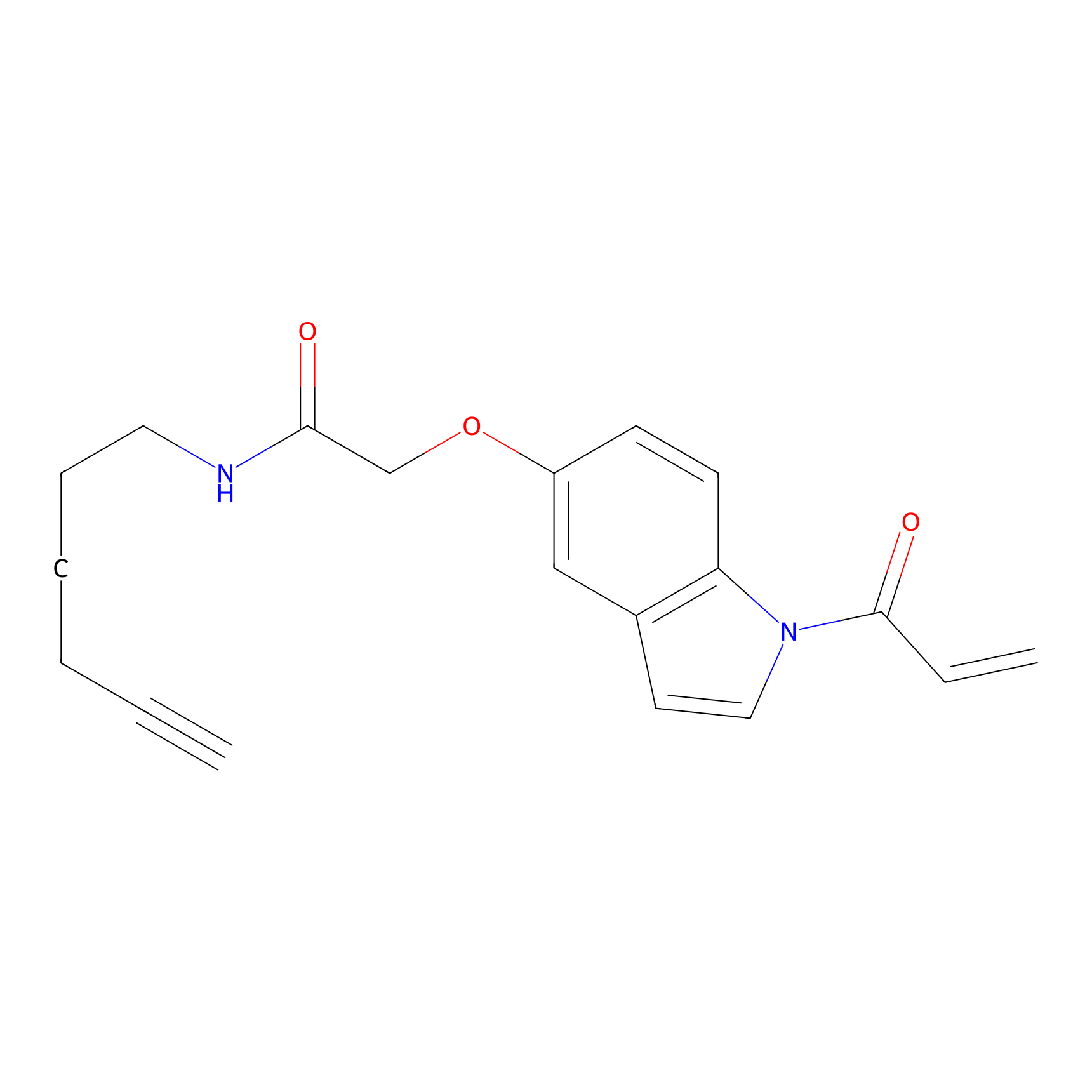 |
C244(0.00); 0.00 | LDD2223 | [4] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0625 | F8 | Ramos | C88(1.09); C134(0.76); C214(1.36) | LDD2187 | [5] |
| LDCM0572 | Fragment10 | Ramos | C88(1.22); C134(1.41); C214(1.07) | LDD2189 | [5] |
| LDCM0573 | Fragment11 | Ramos | C88(0.41); C134(6.81) | LDD2190 | [5] |
| LDCM0574 | Fragment12 | Ramos | C88(0.94); C134(1.05); C214(0.94) | LDD2191 | [5] |
| LDCM0575 | Fragment13 | Ramos | C88(1.26); C134(1.02) | LDD2192 | [5] |
| LDCM0576 | Fragment14 | Ramos | C88(1.15); C134(1.41); C214(0.66) | LDD2193 | [5] |
| LDCM0579 | Fragment20 | Ramos | C88(1.18); C134(1.65); C214(0.86) | LDD2194 | [5] |
| LDCM0580 | Fragment21 | Ramos | C88(0.93); C134(0.99); C214(1.48) | LDD2195 | [5] |
| LDCM0582 | Fragment23 | Ramos | C88(0.85); C134(0.87); C214(1.20) | LDD2196 | [5] |
| LDCM0578 | Fragment27 | Ramos | C88(1.02); C134(0.94) | LDD2197 | [5] |
| LDCM0586 | Fragment28 | Ramos | C88(1.23); C134(0.87) | LDD2198 | [5] |
| LDCM0588 | Fragment30 | Ramos | C88(1.19); C134(0.92); C214(1.57) | LDD2199 | [5] |
| LDCM0589 | Fragment31 | Ramos | C88(1.12); C134(1.09); C214(0.96) | LDD2200 | [5] |
| LDCM0590 | Fragment32 | Ramos | C88(2.19); C134(1.22) | LDD2201 | [5] |
| LDCM0468 | Fragment33 | Ramos | C88(1.07); C134(0.96); C214(1.60) | LDD2202 | [5] |
| LDCM0596 | Fragment38 | Ramos | C88(0.87); C134(0.89) | LDD2203 | [5] |
| LDCM0566 | Fragment4 | Ramos | C88(1.06); C134(1.00); C214(0.65) | LDD2184 | [5] |
| LDCM0610 | Fragment52 | Ramos | C88(1.16); C134(1.23) | LDD2204 | [5] |
| LDCM0614 | Fragment56 | Ramos | C88(1.15); C134(1.20) | LDD2205 | [5] |
| LDCM0569 | Fragment7 | Ramos | C88(1.23); C134(1.13); C214(1.04) | LDD2186 | [5] |
| LDCM0571 | Fragment9 | Ramos | C88(0.82) | LDD2188 | [5] |
| LDCM0022 | KB02 | Ramos | C88(1.47); C214(0.88) | LDD2182 | [5] |
| LDCM0023 | KB03 | Ramos | C88(0.98); C134(1.38); C214(0.85) | LDD2183 | [5] |
| LDCM0024 | KB05 | NALM-6 | C88(2.44); C134(3.09) | LDD3339 | [2] |
| LDCM0170 | Structure8 | Ramos | 6.79 | LDD0433 | [3] |
The Interaction Atlas With This Target
References
