Details of the Probe
General Information of Probe
The Probe Interaction Atlas
Target(s) List of this Probe
|
4 Enzyme Labeled by This Probe
|
||||||||||||||||||||||||||||||||
|
4 Other Labeled by This Probe
|
||||||||||||||||||||||||||||||||
Competitor(s) Related To This Probe
Full Information of The Labelling Profiles of This Probe
Quantification: Probe vs (Probe+Competitor)
Experiment 1 Reporting the Labelling Profiles of This Probe
Probe concentration
Quantitative Method
Competitor Concentration
In Vitro Experiment Model

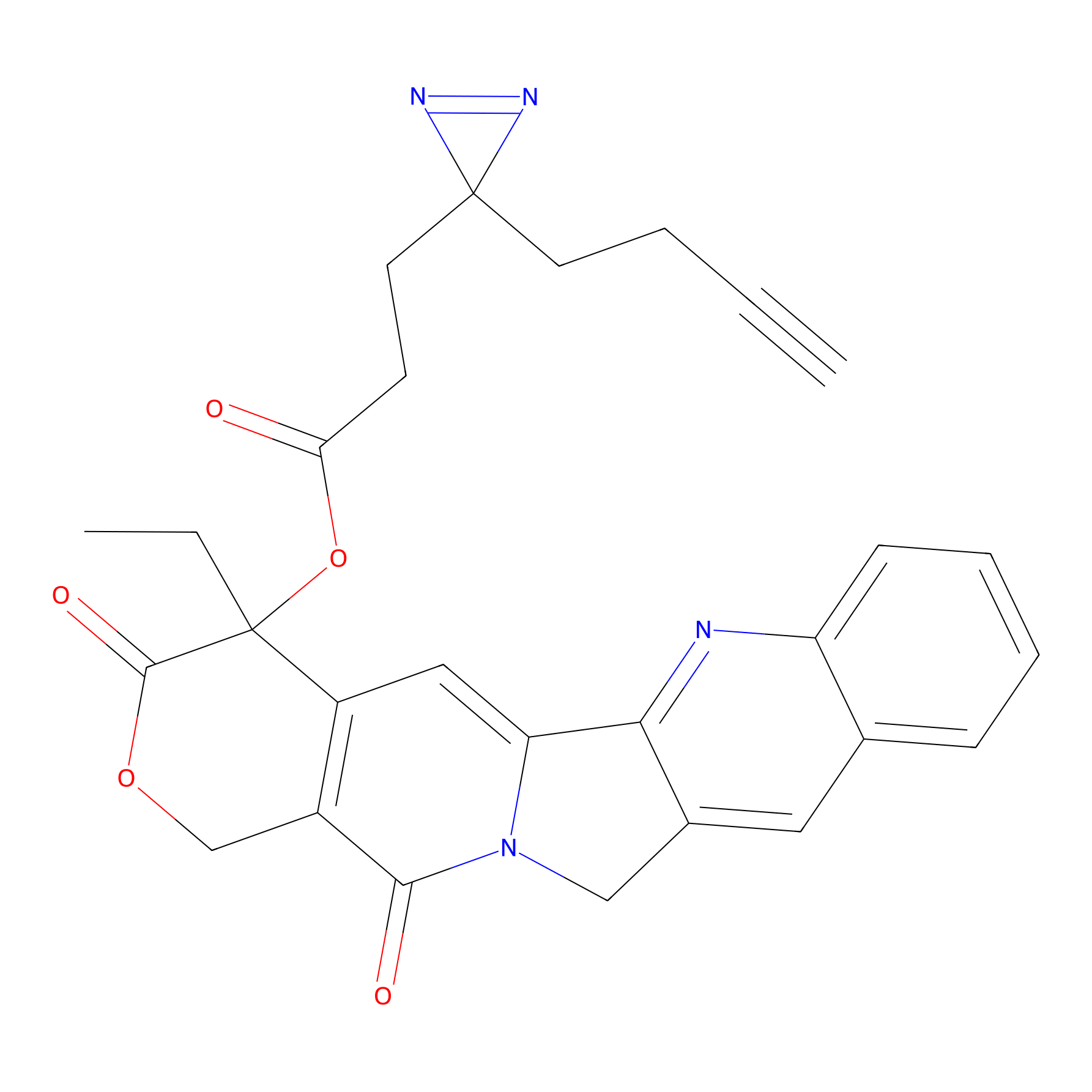


 Download The Altas
Download The Altas