Details of the Target
General Information of Target
| Target ID | LDTP07006 | |||||
|---|---|---|---|---|---|---|
| Target Name | FYVE, RhoGEF and PH domain-containing protein 3 (FGD3) | |||||
| Gene Name | FGD3 | |||||
| Gene ID | 89846 | |||||
| Synonyms |
ZFYVE5; FYVE, RhoGEF and PH domain-containing protein 3; Zinc finger FYVE domain-containing protein 5 |
|||||
| 3D Structure | ||||||
| Sequence |
MESGRGSSTPPGPIAALGMPDTGPGSSSLGKLQALPVGPRAHCGDPVSLAAAGDGSPDIG
PTGELSGSLKIPNRDSGIDSPSSSVAGENFPCEEGLEAGPSPTVLGAHAEMALDSQVPKV TPQEEADSDVGEEPDSENTPQKADKDAGLAQHSGPQKLLHIAQELLHTEETYVKRLHLLD QVFCTRLTDAGIPPEVIMGIFSNISSIHRFHGQFLLPELKTRITEEWDTNPRLGDILQKL APFLKMYGEYVKNFDRAVGLVSTWTQRSPLFKDVVHSIQKQEVCGNLTLQHHMLEPVQRV PRYELLLKDYLKRLPQDAPDRKDAERSLELISTAANHSNAAIRKVEKMHKLLEVYEQLGG EEDIVNPANELIKEGQIQKLSAKNGTPQDRHLFLFNSMILYCVPKLRLMGQKFSVREKMD ISGLQVQDIVKPNTAHTFIITGRKRSLELQTRTEEEKKEWIQIIQATIEKHKQNSETFKA FGGAFSQDEDPSLSPDMPITSTSPVEPVVTTEGSSGAAGLEPRKLSSKTRRDKEKQSCKS CGETFNSITKRRHHCKLCGAVICGKCSEFKAENSRQSRVCRDCFLTQPVAPESTEKTPTA DPQPSLLCGPLRLSESGETWSEVWAAIPMSDPQVLHLQGGSQDGRLPRTIPLPSCKLSVP DPEERLDSGHVWKLQWAKQSWYLSASSAELQQQWLETLSTAAHGDTAQDSPGALQLQVPM GAAAP |
|||||
| Target Bioclass |
Other
|
|||||
| Subcellular location |
Cytoplasm
|
|||||
| Function |
Promotes the formation of filopodia. May activate CDC42, a member of the Ras-like family of Rho- and Rac proteins, by exchanging bound GDP for free GTP. Plays a role in regulating the actin cytoskeleton and cell shape.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Target Site Mutations in Different Cell Lines
| Cell line | Mutation details | Probe for labeling this protein in this cell | |||
|---|---|---|---|---|---|
| COLO678 | SNV: p.G4V | DBIA Probe Info | |||
| G415 | SNV: p.H208Q | DBIA Probe Info | |||
| HCT15 | Deletion: p.R302GfsTer10 | . | |||
| JHH7 | SNV: p.V261L | . | |||
| JURKAT | SNV: p.A435T; p.Q715Ter | . | |||
| MDAMB231 | SNV: p.P81S | . | |||
| MFE319 | SNV: p.H291Y | . | |||
| MOLT4 | SNV: p.L424F; p.K533N; p.E696Ter; p.A702S | IA-alkyne Probe Info | |||
| SKES1 | SNV: p.G410C | . | |||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
DBIA Probe Info |
 |
C655(52.44) | LDD0209 | [1] | |
|
4-Iodoacetamidophenylacetylene Probe Info |
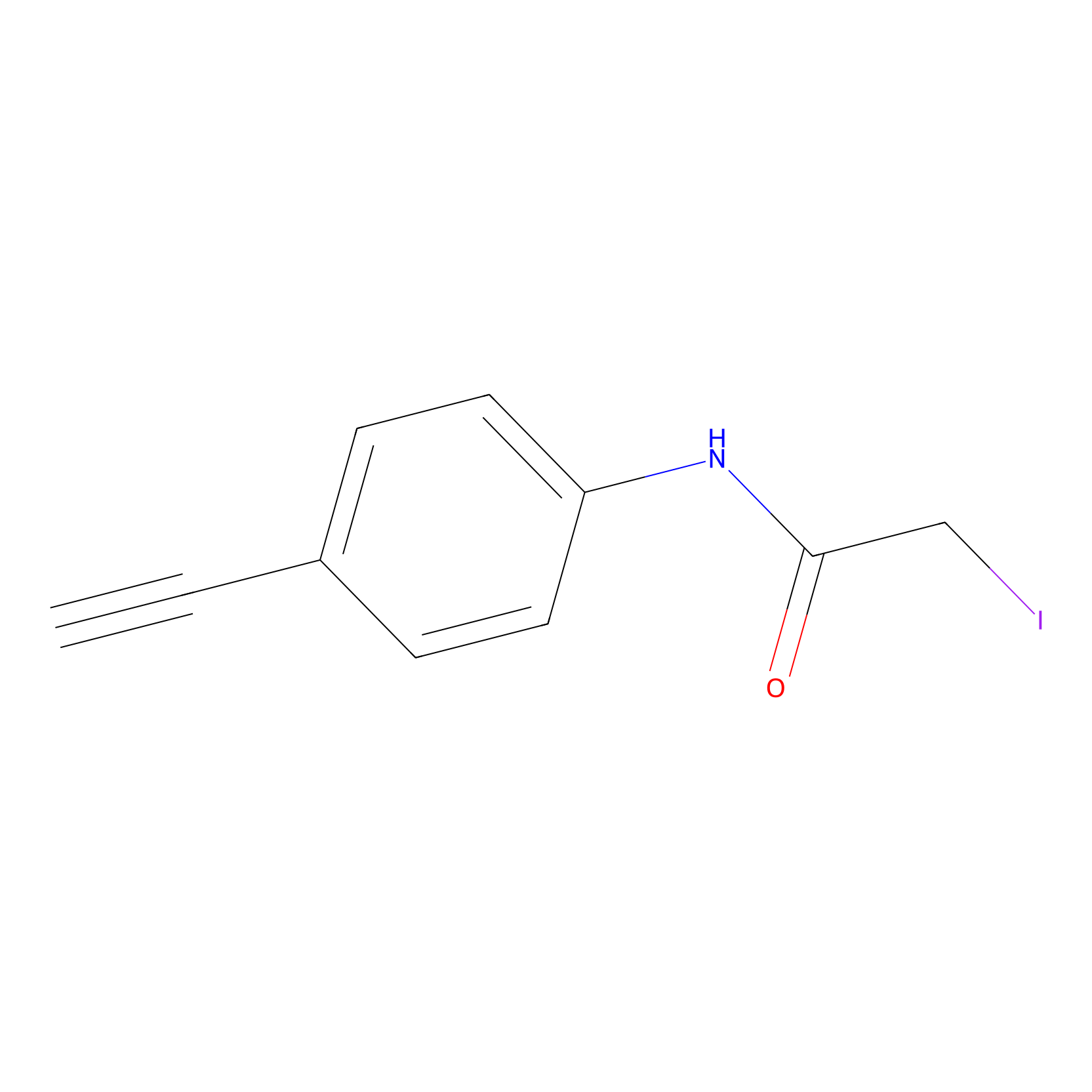 |
C184(0.00); C608(0.00); C541(0.00); C655(0.00) | LDD0038 | [2] | |
|
IA-alkyne Probe Info |
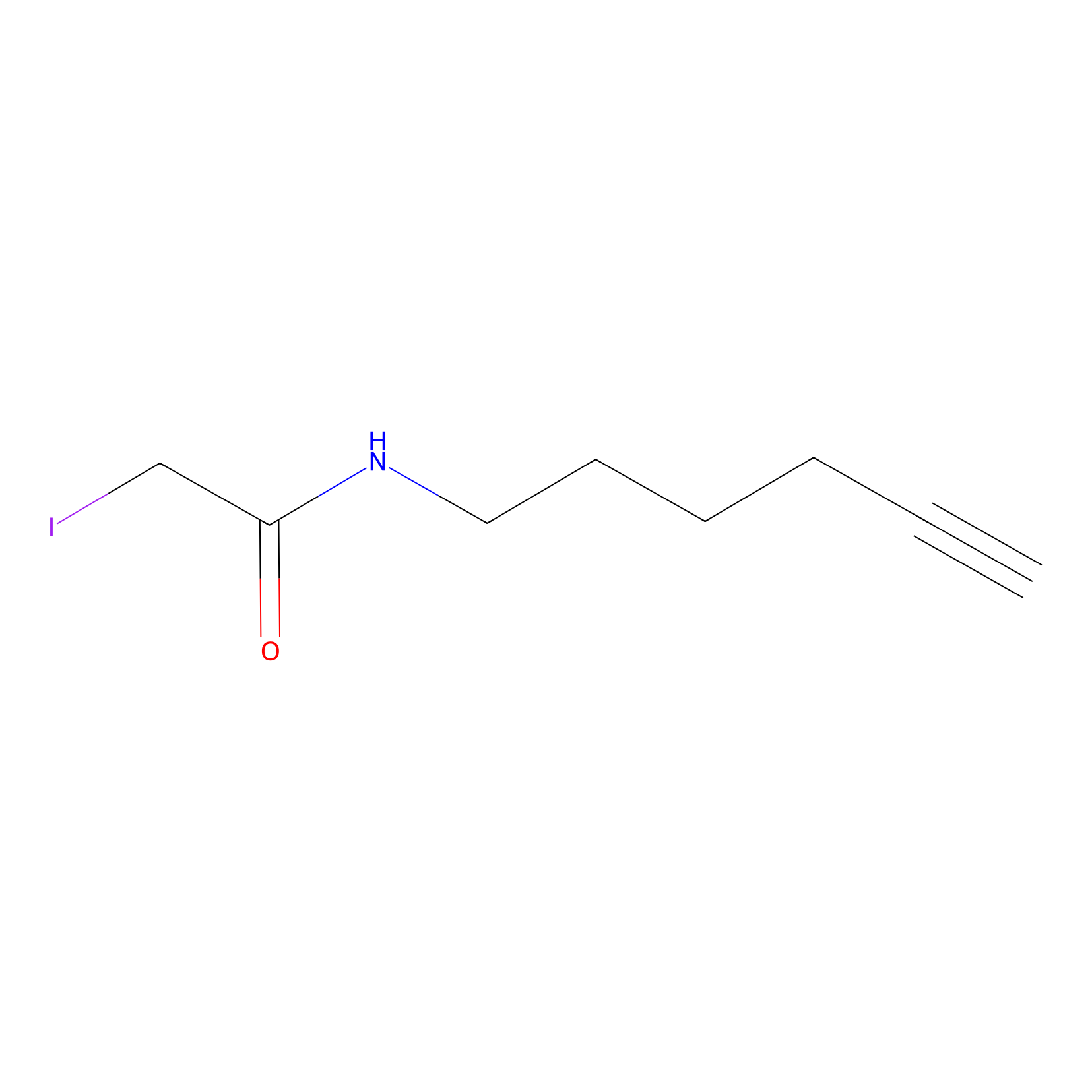 |
C184(0.00); C541(0.00); C655(0.00) | LDD0036 | [2] | |
|
Lodoacetamide azide Probe Info |
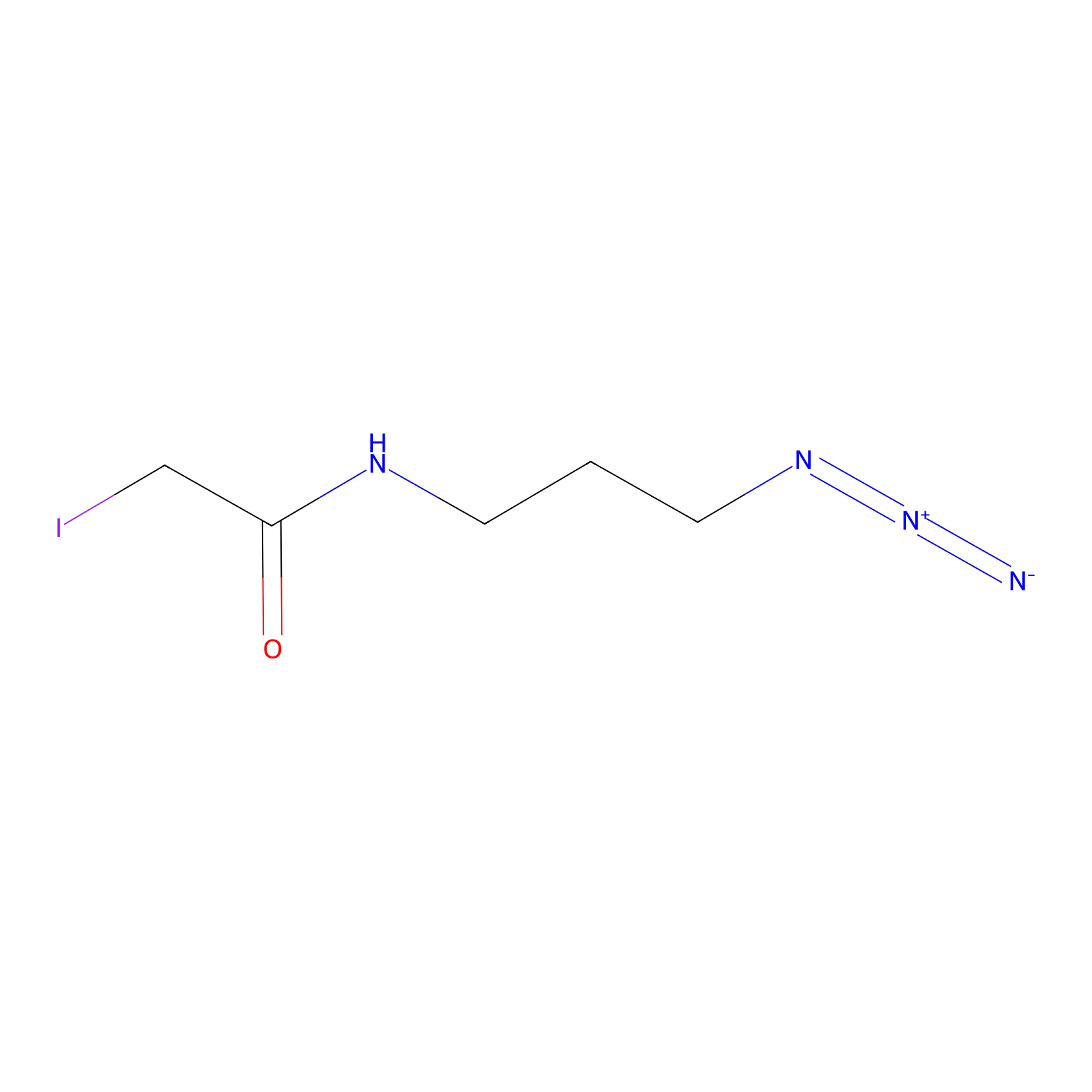 |
C583(0.00); C184(0.00); C541(0.00) | LDD0037 | [2] | |
|
NAIA_5 Probe Info |
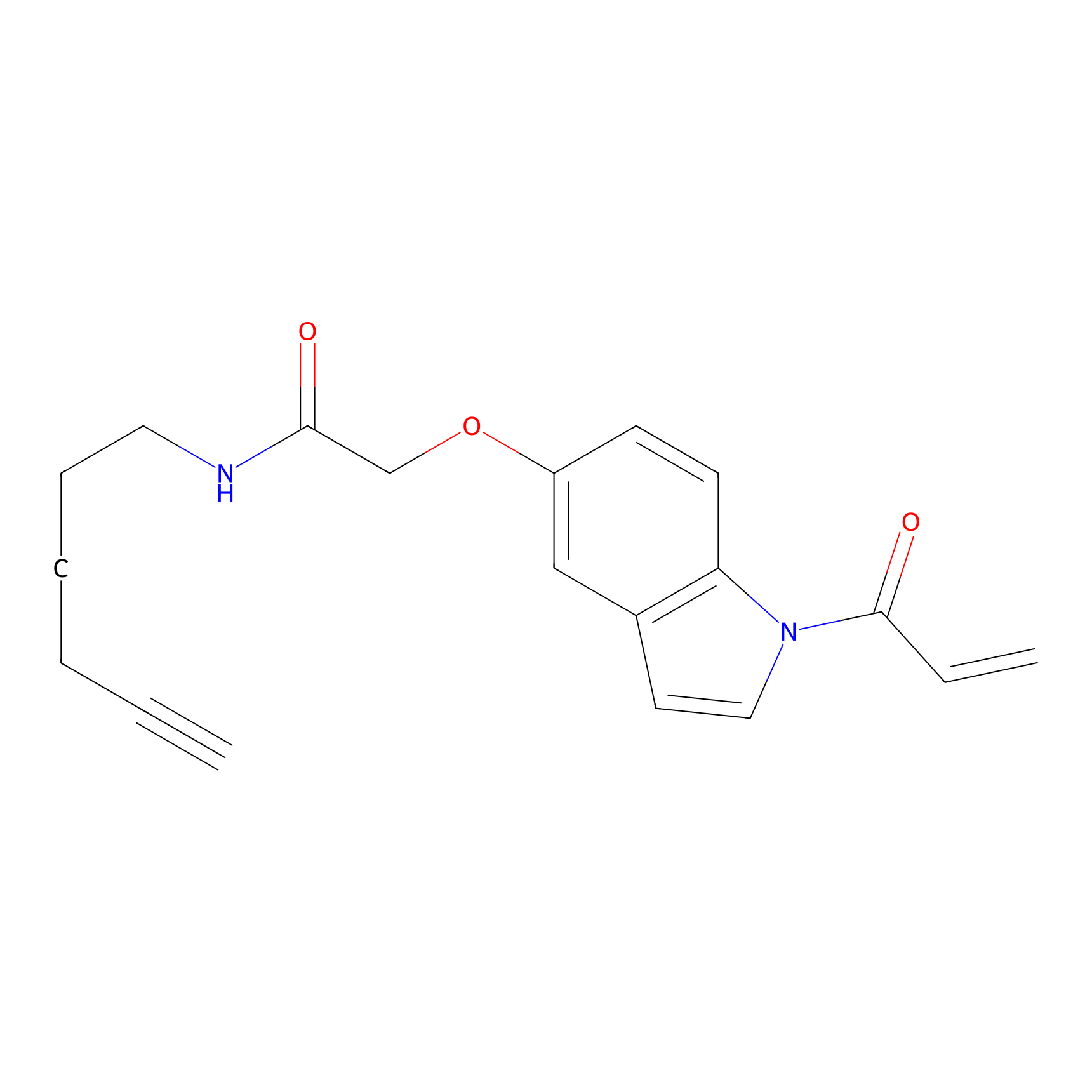 |
N.A. | LDD2224 | [3] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0625 | F8 | Ramos | C184(1.14); C43(1.19) | LDD2187 | [4] |
| LDCM0572 | Fragment10 | Ramos | C184(1.06); C43(1.27); C655(0.97) | LDD2189 | [4] |
| LDCM0573 | Fragment11 | Ramos | C184(4.42); C43(0.35); C655(0.24) | LDD2190 | [4] |
| LDCM0574 | Fragment12 | Ramos | C184(4.36); C43(1.02); C655(0.78) | LDD2191 | [4] |
| LDCM0575 | Fragment13 | Ramos | C184(0.92); C43(0.63) | LDD2192 | [4] |
| LDCM0576 | Fragment14 | Ramos | C184(4.88); C43(1.12) | LDD2193 | [4] |
| LDCM0579 | Fragment20 | Ramos | C184(3.41); C43(0.86); C655(0.84) | LDD2194 | [4] |
| LDCM0580 | Fragment21 | Ramos | C184(0.86); C43(0.87); C655(0.69) | LDD2195 | [4] |
| LDCM0582 | Fragment23 | Ramos | C184(1.36); C43(1.15); C655(0.46) | LDD2196 | [4] |
| LDCM0578 | Fragment27 | Ramos | C184(0.76); C43(1.32); C655(0.89) | LDD2197 | [4] |
| LDCM0586 | Fragment28 | Ramos | C184(1.86); C43(1.57); C655(0.97) | LDD2198 | [4] |
| LDCM0588 | Fragment30 | Ramos | C184(0.84); C43(1.13); C655(0.93) | LDD2199 | [4] |
| LDCM0589 | Fragment31 | Ramos | C184(1.75); C43(1.04); C655(0.74) | LDD2200 | [4] |
| LDCM0590 | Fragment32 | Ramos | C184(2.42); C43(1.53) | LDD2201 | [4] |
| LDCM0468 | Fragment33 | Ramos | C184(0.85); C43(1.17); C655(0.84) | LDD2202 | [4] |
| LDCM0596 | Fragment38 | Ramos | C184(1.67); C43(0.93); C655(0.79) | LDD2203 | [4] |
| LDCM0566 | Fragment4 | Ramos | C184(1.85); C43(1.27); C655(0.43) | LDD2184 | [4] |
| LDCM0610 | Fragment52 | Ramos | C184(1.23); C43(1.73) | LDD2204 | [4] |
| LDCM0614 | Fragment56 | Ramos | C184(0.83); C43(0.94); C655(1.07) | LDD2205 | [4] |
| LDCM0569 | Fragment7 | Ramos | C184(1.97); C43(1.15); C655(0.58) | LDD2186 | [4] |
| LDCM0571 | Fragment9 | Ramos | C184(2.01); C43(1.20) | LDD2188 | [4] |
| LDCM0022 | KB02 | Ramos | C184(2.61); C43(1.35); C655(0.63) | LDD2182 | [4] |
| LDCM0023 | KB03 | Jurkat | C655(52.44) | LDD0209 | [1] |
| LDCM0024 | KB05 | HMCB | C655(2.15) | LDD3312 | [5] |
| LDCM0131 | RA190 | MM1.R | C43(1.30) | LDD0304 | [6] |
References
