Details of the Target
General Information of Target
| Target ID | LDTP00840 | |||||
|---|---|---|---|---|---|---|
| Target Name | Phospholipid phosphatase 2 (PLPP2) | |||||
| Gene Name | PLPP2 | |||||
| Gene ID | 8612 | |||||
| Synonyms |
LPP2; PPAP2C; Phospholipid phosphatase 2; EC 3.1.3.-; EC 3.1.3.4; Lipid phosphate phosphohydrolase 2; PAP2-gamma; PAP2-G; Phosphatidate phosphohydrolase type 2c; Phosphatidic acid phosphatase 2c; PAP-2c; PAP2c
|
|||||
| 3D Structure | ||||||
| Sequence |
MQRRWVFVLLDVLCLLVASLPFAILTLVNAPYKRGFYCGDDSIRYPYRPDTITHGLMAGV
TITATVILVSAGEAYLVYTDRLYSRSDFNNYVAAVYKVLGTFLFGAAVSQSLTDLAKYMI GRLRPNFLAVCDPDWSRVNCSVYVQLEKVCRGNPADVTEARLSFYSGHSSFGMYCMVFLA LYVQARLCWKWARLLRPTVQFFLVAFALYVGYTRVSDYKHHWSDVLVGLLQGALVAALTV CYISDFFKARPPQHCLKEEELERKPSLSLTLTLGEADHNHYGYPHSSS |
|||||
| Target Bioclass |
Enzyme
|
|||||
| Family |
PA-phosphatase related phosphoesterase family
|
|||||
| Subcellular location |
Membrane
|
|||||
| Function |
Magnesium-independent phospholipid phosphatase that catalyzes the dephosphorylation of a variety of glycerolipid and sphingolipid phosphate esters including phosphatidate/PA, lysophosphatidate/LPA, sphingosine 1-phosphate/S1P and ceramide 1-phosphate/C1P. Has no apparent extracellular phosphatase activity and therefore most probably acts intracellularly. Also acts on N-oleoyl ethanolamine phosphate/N-(9Z-octadecenoyl)-ethanolamine phosphate, a potential physiological compound. Through dephosphorylation of these bioactive lipid mediators produces new bioactive compounds and may regulate signal transduction in different cellular processes (Probable). Indirectly regulates, for instance, cell cycle G1/S phase transition through its phospholipid phosphatase activity.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
m-APA Probe Info |
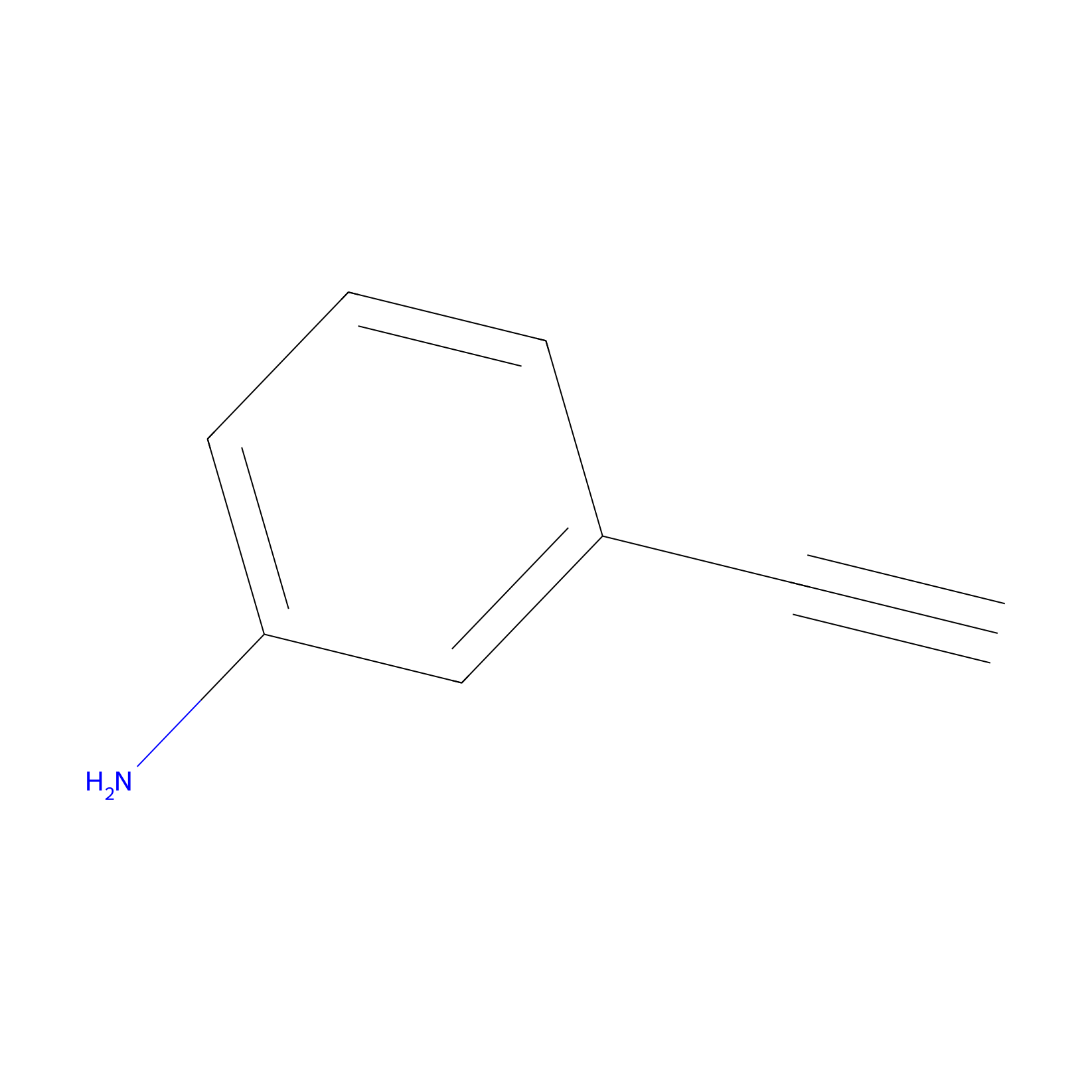 |
9.97 | LDD0402 | [1] | |
|
DBIA Probe Info |
 |
C276(2.50) | LDD3311 | [2] | |
|
BTD Probe Info |
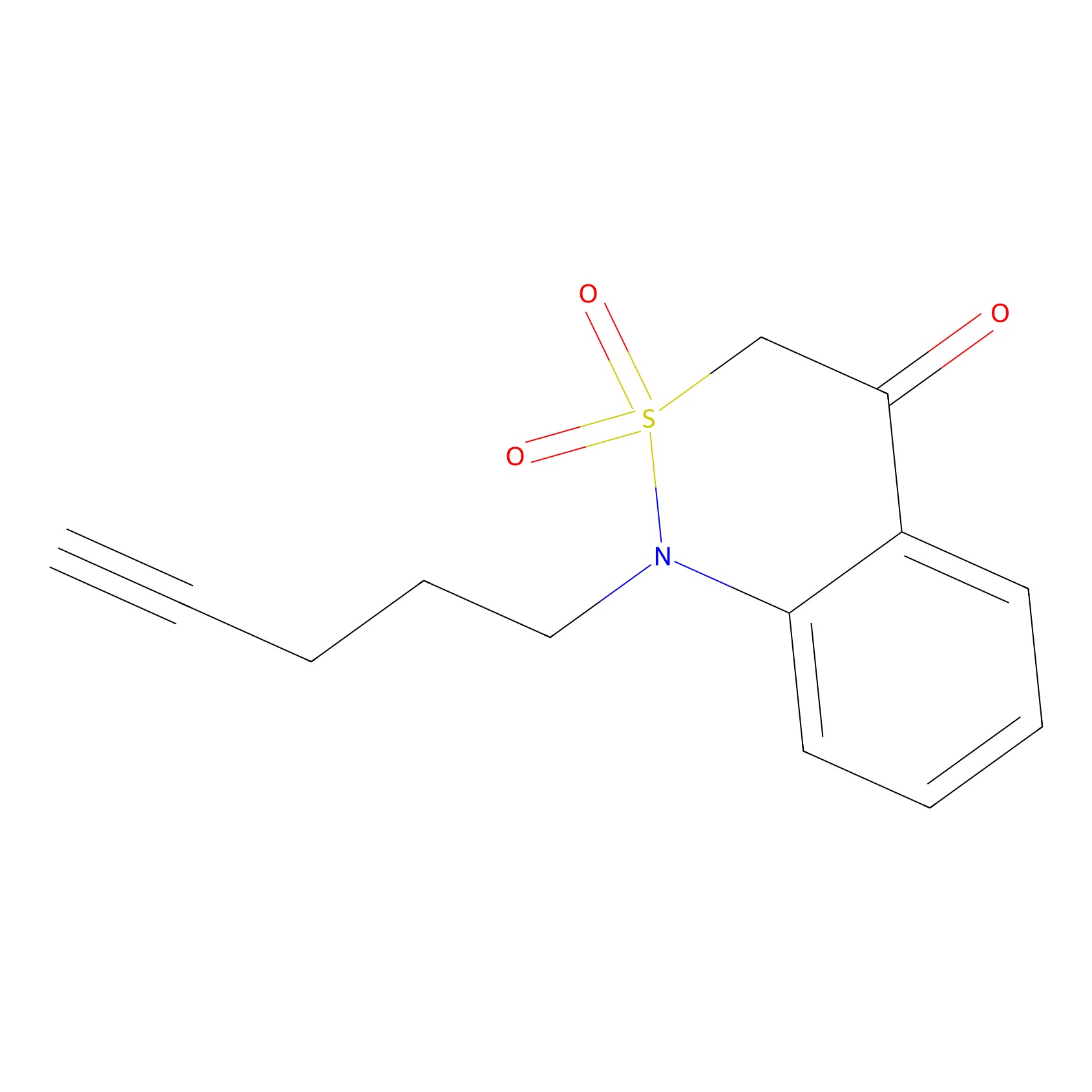 |
C38(1.00) | LDD2099 | [3] | |
|
AHL-Pu-1 Probe Info |
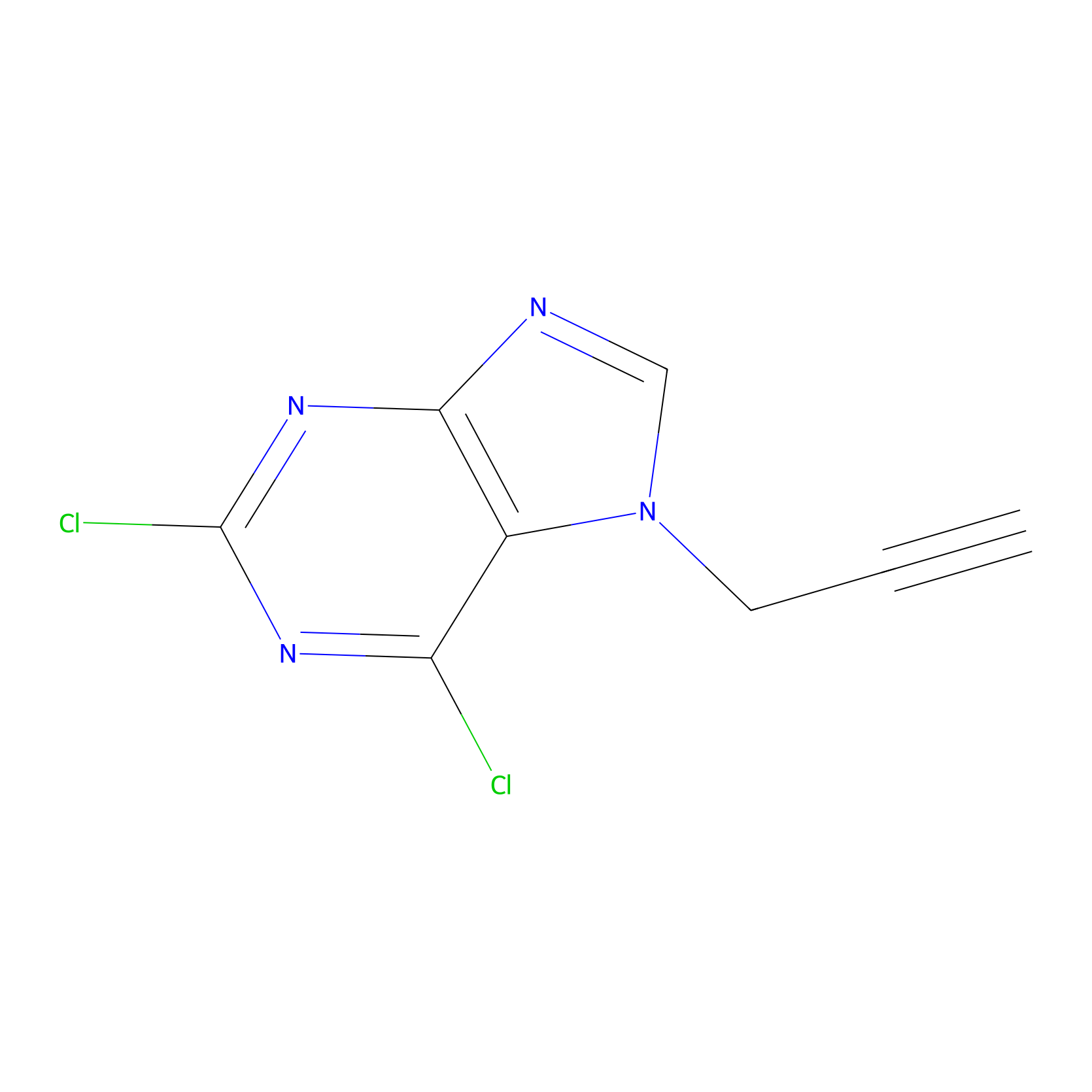 |
C255(4.54) | LDD0171 | [4] | |
|
TFBX Probe Info |
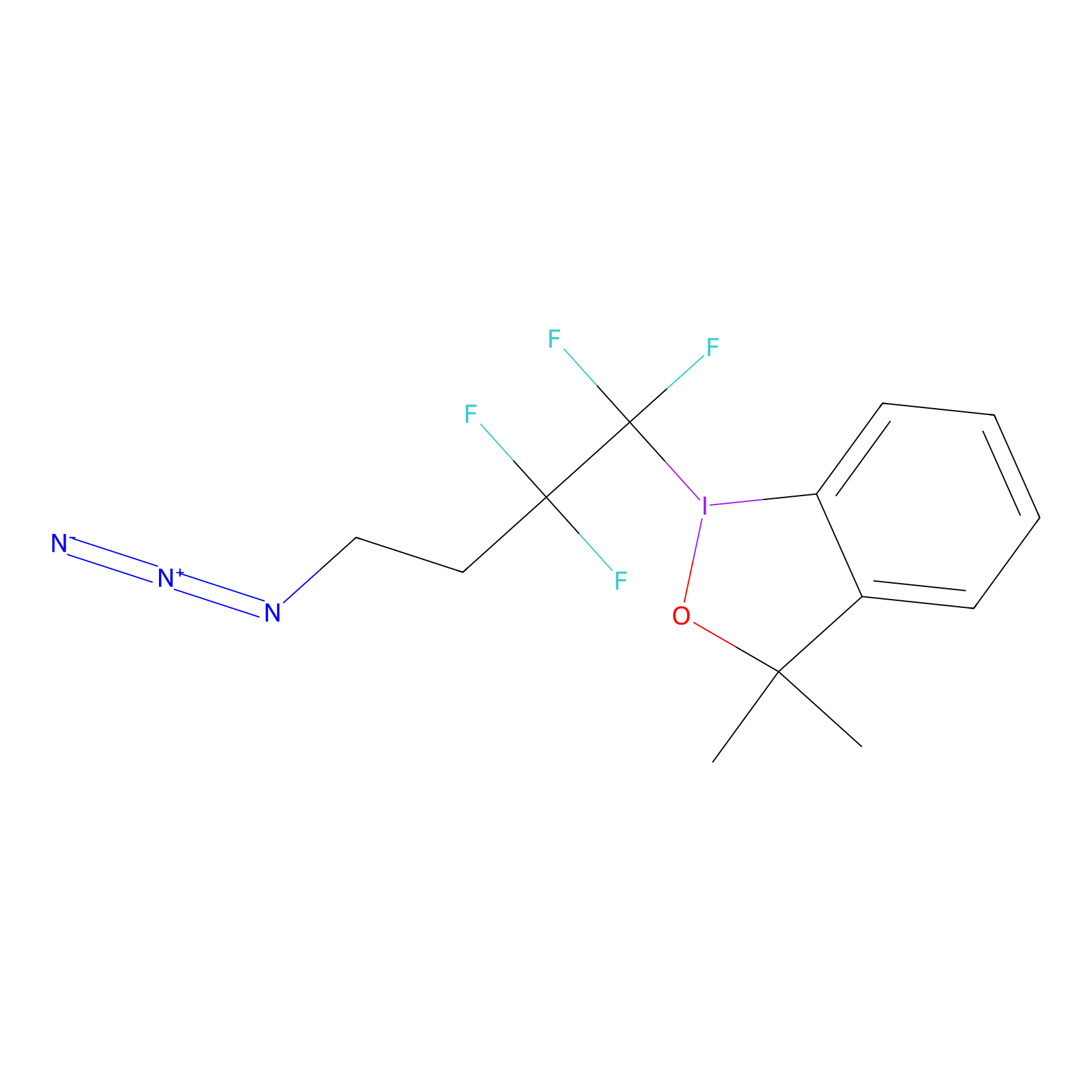 |
N.A. | LDD0148 | [5] | |
|
NAIA_5 Probe Info |
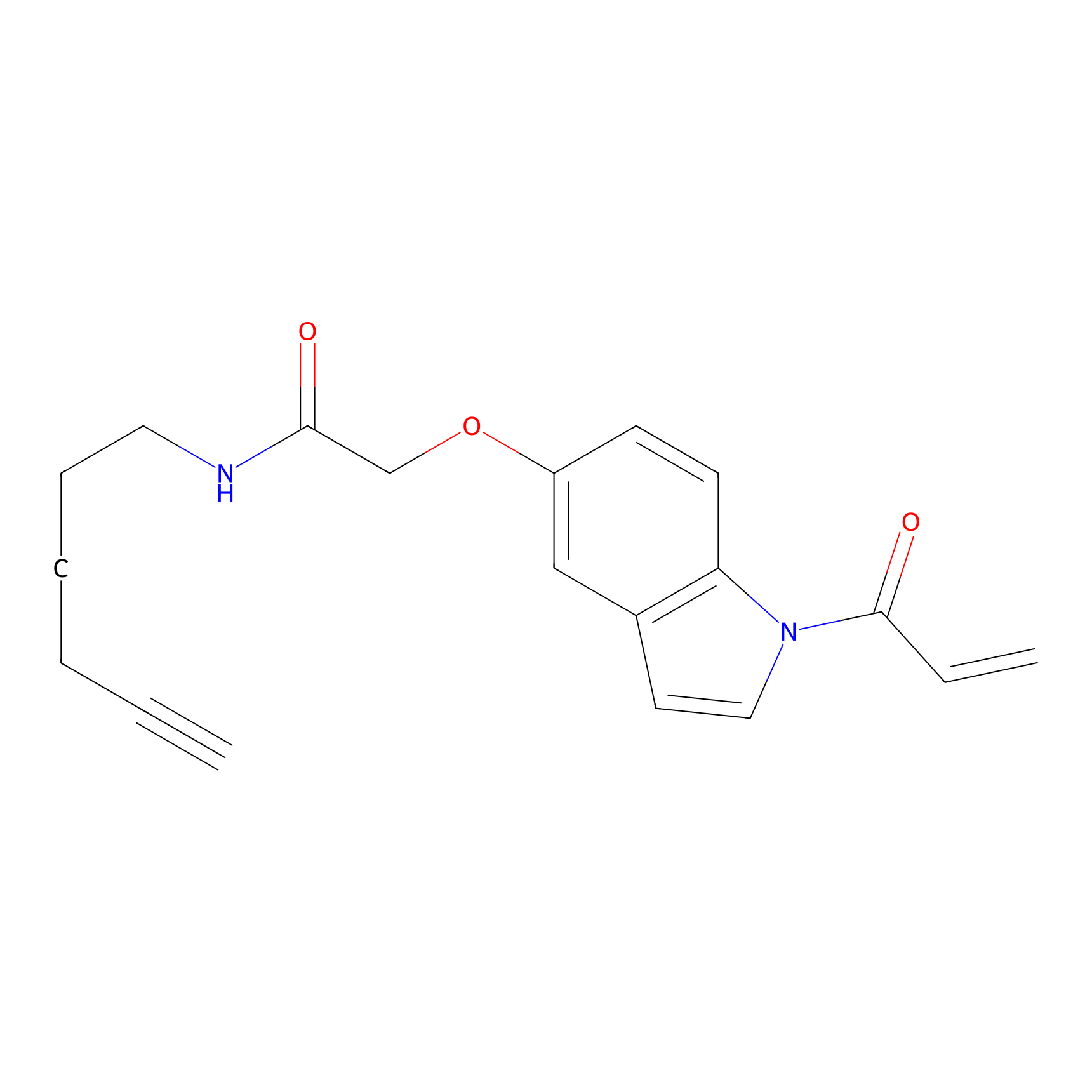 |
N.A. | LDD2223 | [6] | |
PAL-AfBPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
STS-1 Probe Info |
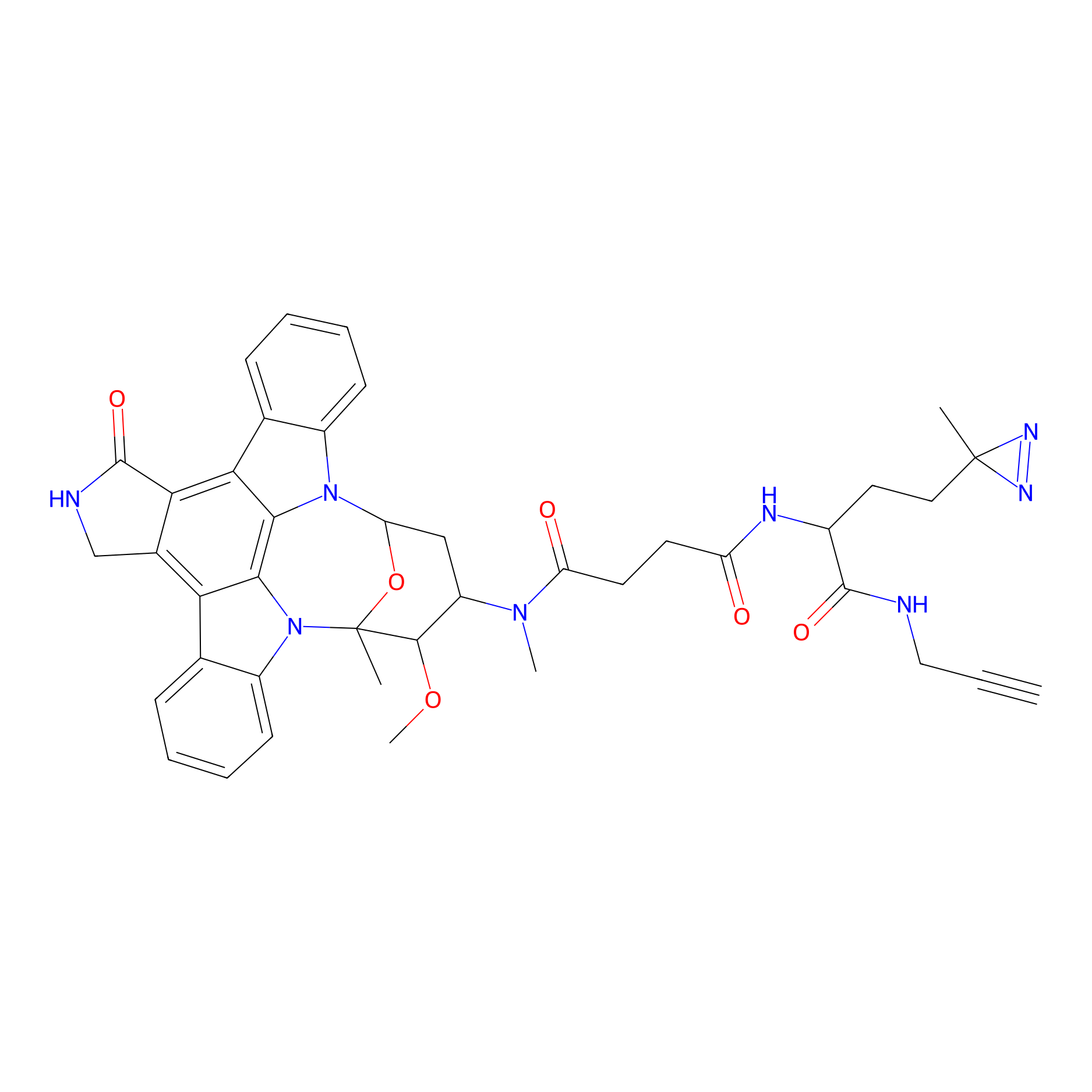 |
N.A. | LDD0136 | [7] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0548 | 1-(4-(Benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)-2-nitroethan-1-one | MDA-MB-231 | C255(0.73) | LDD2142 | [3] |
| LDCM0026 | 4SU-RNA+native RNA | DM93 | C255(4.54) | LDD0171 | [4] |
| LDCM0156 | Aniline | NCI-H1299 | 11.69 | LDD0403 | [1] |
| LDCM0022 | KB02 | AGS | C276(1.70) | LDD2263 | [2] |
| LDCM0023 | KB03 | A-375 | C276(2.27) | LDD2672 | [2] |
| LDCM0024 | KB05 | G361 | C276(2.50) | LDD3311 | [2] |
| LDCM0506 | Nucleophilic fragment 16a | MDA-MB-231 | C38(1.00) | LDD2099 | [3] |
| LDCM0514 | Nucleophilic fragment 20a | MDA-MB-231 | C38(0.94) | LDD2107 | [3] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Transporter and channel
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Translocating chain-associated membrane protein 1-like 1 (TRAM1L1) | TRAM family | Q8N609 | |||
Other
References
