Details of the Target
General Information of Target
| Target ID | LDTP14185 | |||||
|---|---|---|---|---|---|---|
| Target Name | Beta-secretase 2 (BACE2) | |||||
| Gene Name | BACE2 | |||||
| Gene ID | 25825 | |||||
| Synonyms |
AEPLC; ALP56; ASP21; Beta-secretase 2; EC 3.4.23.45; Aspartic-like protease 56 kDa; Aspartyl protease 1; ASP1; Asp 1; Beta-site amyloid precursor protein cleaving enzyme 2; Beta-site APP cleaving enzyme 2; Down region aspartic protease; DRAP; Memapsin-1; Membrane-associated aspartic protease 1; Theta-secretase
|
|||||
| 3D Structure | ||||||
| Sequence |
MEFPIGSLETNNFRRFTPESLVEIEKQIAAKQGTKKAREKHREQKDQEEKPRPQLDLKAC
NQLPKFYGELPAELIGEPLEDLDPFYSTHRTFMVLNKGRTISRFSATRALWLFSPFNLIR RTAIKVSVHSWFSLFITVTILVNCVCMTRTDLPEKIEYVFTVIYTFEALIKILARGFCLN EFTYLRDPWNWLDFSVITLAYVGTAIDLRGISGLRTFRVLRALKTVSVIPGLKVIVGALI HSVKKLADVTILTIFCLSVFALVGLQLFKGNLKNKCVKNDMAVNETTNYSSHRKPDIYIN KRGTSDPLLCGNGSDSGHCPDGYICLKTSDNPDFNYTSFDSFAWAFLSLFRLMTQDSWER LYQQTLRTSGKIYMIFFVLVIFLGSFYLVNLILAVVTMAYEEQNQATTDEIEAKEKKFQE ALEMLRKEQEVLAALGIDTTSLHSHNGSPLTSKNASERRHRIKPRVSEGSTEDNKSPRSD PYNQRRMSFLGLASGKRRASHGSVFHFRSPGRDISLPEGVTDDGVFPGDHESHRGSLLLG GGAGQQGPLPRSPLPQPSNPDSRHGEDEHQPPPTSELAPGAVDVSAFDAGQKKTFLSAEY LDEPFRAQRAMSVVSIITSVLEELEESEQKCPPCLTSLSQKYLIWDCCPMWVKLKTILFG LVTDPFAELTITLCIVVNTIFMAMEHHGMSPTFEAMLQIGNIVFTIFFTAEMVFKIIAFD PYYYFQKKWNIFDCIIVTVSLLELGVAKKGSLSVLRSFRLLRVFKLAKSWPTLNTLIKII GNSVGALGNLTIILAIIVFVFALVGKQLLGENYRNNRKNISAPHEDWPRWHMHDFFHSFL IVFRILCGEWIENMWACMEVGQKSICLILFLTVMVLGNLVVLNLFIALLLNSFSADNLTA PEDDGEVNNLQVALARIQVFGHRTKQALCSFFSRSCPFPQPKAEPELVVKLPLSSSKAEN HIAANTARGSSGGLQAPRGPRDEHSDFIANPTVWVSVPIAEGESDLDDLEDDGGEDAQSF QQEVIPKGQQEQLQQVERCGDHLTPRSPGTGTSSEDLAPSLGETWKDESVPQVPAEGVDD TSSSEGSTVDCLDPEEILRKIPELADDLEEPDDCFTEGCIRHCPCCKLDTTKSPWDVGWQ VRKTCYRIVEHSWFESFIIFMILLSSGSLAFEDYYLDQKPTVKALLEYTDRVFTFIFVFE MLLKWVAYGFKKYFTNAWCWLDFLIVNISLISLTAKILEYSEVAPIKALRTLRALRPLRA LSRFEGMRVVVDALVGAIPSIMNVLLVCLIFWLIFSIMGVNLFAGKFWRCINYTDGEFSL VPLSIVNNKSDCKIQNSTGSFFWVNVKVNFDNVAMGYLALLQVATFKGWMDIMYAAVDSR EVNMQPKWEDNVYMYLYFVIFIIFGGFFTLNLFVGVIIDNFNQQKKKLGGQDIFMTEEQK KYYNAMKKLGSKKPQKPIPRPLNKFQGFVFDIVTRQAFDITIMVLICLNMITMMVETDDQ SEEKTKILGKINQFFVAVFTGECVMKMFALRQYYFTNGWNVFDFIVVVLSIASLIFSAIL KSLQSYFSPTLFRVIRLARIGRILRLIRAAKGIRTLLFALMMSLPALFNIGLLLFLVMFI YSIFGMSSFPHVRWEAGIDDMFNFQTFANSMLCLFQITTSAGWDGLLSPILNTGPPYCDP NLPNSNGTRGDCGSPAVGIIFFTTYIIISFLIMVNMYIAVILENFNVATEESTEPLSEDD FDMFYETWEKFDPEATQFITFSALSDFADTLSGPLRIPKPNRNILIQMDLPLVPGDKIHC LDILFAFTKNVLGESGELDSLKANMEEKFMATNLSKSSYEPIATTLRWKQEDISATVIQK AYRSYVLHRSMALSNTPCVPRAEEEAASLPDEGFVAFTANENCVLPDKSETASATSFPPS YESVTRGLSDRVNMRTSSSIQNEDEATSMELIAPGP |
|||||
| Target Type |
Clinical trial
|
|||||
| Target Bioclass |
Enzyme
|
|||||
| Family |
Peptidase A1 family
|
|||||
| Subcellular location |
Cell membrane
|
|||||
| Function |
Responsible for the proteolytic processing of the amyloid precursor protein (APP). Cleaves APP, between residues 690 and 691, leading to the generation and extracellular release of beta-cleaved soluble APP, and a corresponding cell-associated C-terminal fragment which is later released by gamma-secretase. It has also been shown that it can cleave APP between residues 671 and 672. Involved in the proteolytic shedding of PMEL at early stages of melanosome biogenesis. Cleaves PMEL within the M-beta fragment to release the amyloidogenic PMEL luminal fragment containing M-alpha and a small portion of M-beta N-terminus. This is a prerequisite step for subsequent processing and assembly of PMEL fibrils into amyloid sheets. Responsible also for the proteolytic processing of CLTRN in pancreatic beta cells.
|
|||||
| TTD ID | ||||||
| Uniprot ID | ||||||
| DrugMap ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
| ChEMBL ID | ||||||
Target Site Mutations in Different Cell Lines
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
OPA-S-S-alkyne Probe Info |
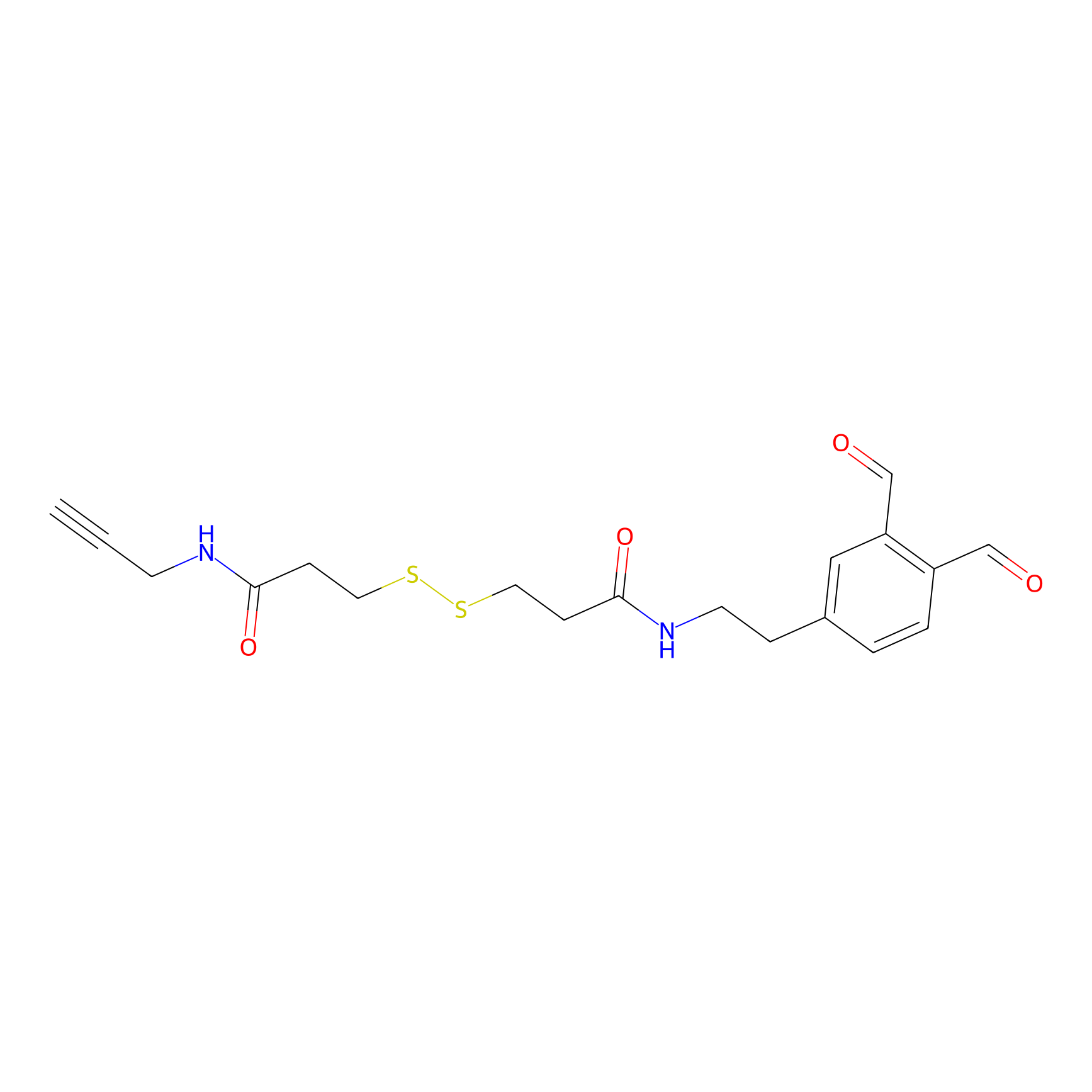 |
K299(1.18) | LDD3494 | [1] | |
