Details of the Target
General Information of Target
| Target ID | LDTP11790 | |||||
|---|---|---|---|---|---|---|
| Target Name | Sphingosine 1-phosphate receptor 5 (S1PR5) | |||||
| Gene Name | S1PR5 | |||||
| Gene ID | 53637 | |||||
| Synonyms |
EDG8; Sphingosine 1-phosphate receptor 5; S1P receptor 5; S1P5; Endothelial differentiation G-protein-coupled receptor 8; Sphingosine 1-phosphate receptor Edg-8; S1P receptor Edg-8 |
|||||
| 3D Structure | ||||||
| Sequence |
MAFPAGFGWAAATAAYQVEGGWDADGKGPCVWDTFTHQGGERVFKNQTGDVACGSYTLWE
EDLKCIKQLGLTHYRFSLSWSRLLPDGTTGFINQKGIDYYNKIIDDLLKNGVTPIVTLYH FDLPQTLEDQGGWLSEAIIESFDKYAQFCFSTFGDRVKQWITINEANVLSVMSYDLGMFP PGIPHFGTGGYQAAHNLIKAHARSWHSYDSLFRKKQKGMVSLSLFAVWLEPADPNSVSDQ EAAKRAITFHLDLFAKPIFIDGDYPEVVKSQIASMSQKQGYPSSRLPEFTEEEKKMIKGT ADFFAVQYYTTRLIKYQENKKGELGILQDAEIEFFPDPSWKNVDWIYVVPWGVCKLLKYI KDTYNNPVIYITENGFPQSDPAPLDDTQRWEYFRQTFQELFKAIQLDKVNLQVYCAWSLL DNFEWNQGYSSRFGLFHVDFEDPARPRVPYTSAKEYAKIIRNNGLEAHL |
|||||
| Target Type |
Clinical trial
|
|||||
| Target Bioclass |
GPCR
|
|||||
| Family |
G-protein coupled receptor 1 family
|
|||||
| Subcellular location |
Cell membrane
|
|||||
| Function |
Receptor for the lysosphingolipid sphingosine 1-phosphate (S1P). S1P is a bioactive lysophospholipid that elicits diverse physiological effect on most types of cells and tissues. Is coupled to both the G(i/0)alpha and G(12) subclass of heteromeric G-proteins. May play a regulatory role in the transformation of radial glial cells into astrocytes and may affect proliferative activity of these cells.
|
|||||
| TTD ID | ||||||
| Uniprot ID | ||||||
| DrugMap ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
| ChEMBL ID | ||||||
Target Site Mutations in Different Cell Lines
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
BTD Probe Info |
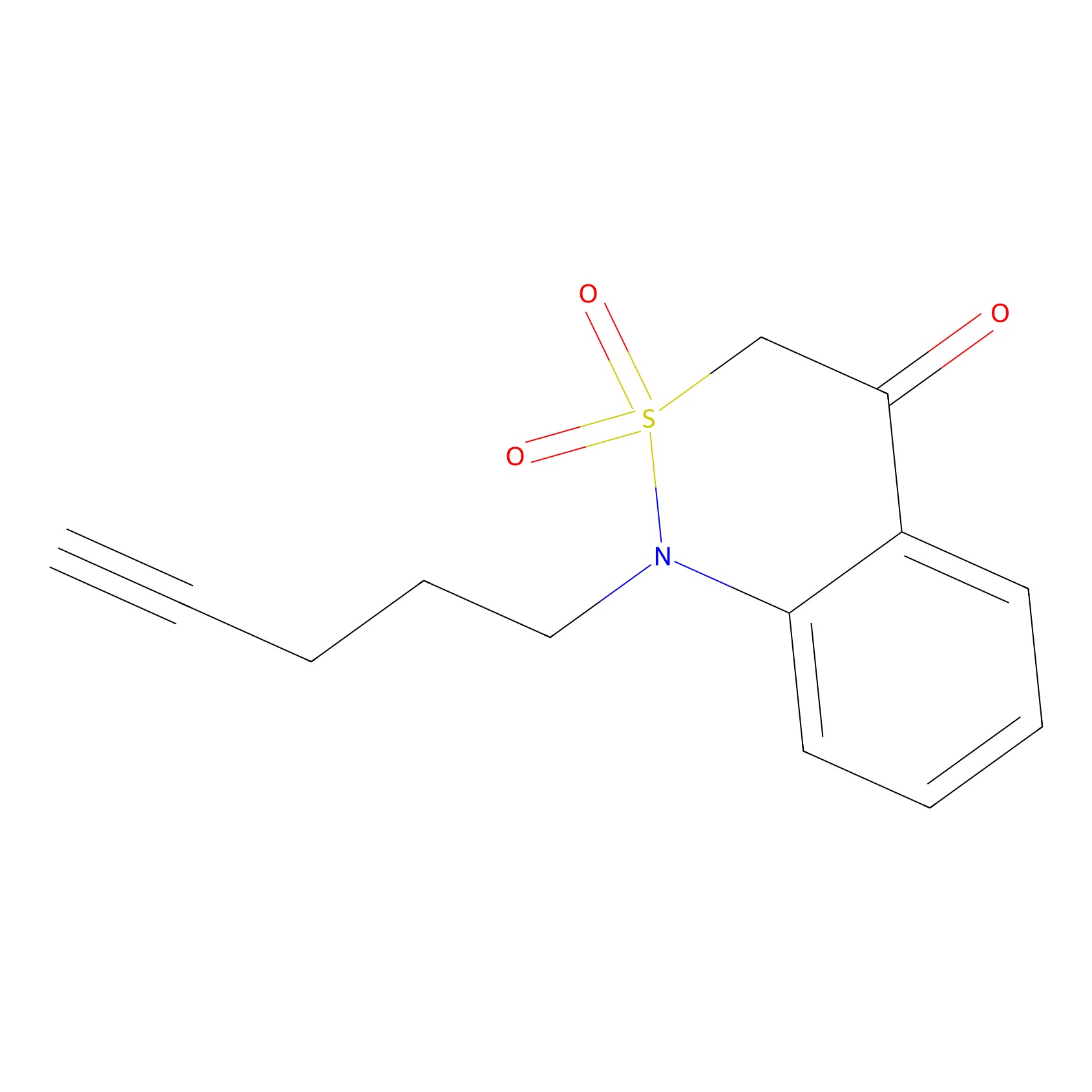 |
C323(0.82) | LDD2100 | [1] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0548 | 1-(4-(Benzo[d][1,3]dioxol-5-ylmethyl)piperazin-1-yl)-2-nitroethan-1-one | MDA-MB-231 | C322(0.81) | LDD2142 | [1] |
| LDCM0519 | 1-(6-methoxy-3,4-dihydroquinolin-1(2H)-yl)-2-nitroethan-1-one | MDA-MB-231 | C322(0.91) | LDD2112 | [1] |
| LDCM0507 | Nucleophilic fragment 16b | MDA-MB-231 | C323(0.82) | LDD2100 | [1] |
