Details of the Target
General Information of Target
| Target ID | LDTP03757 | |||||
|---|---|---|---|---|---|---|
| Target Name | Fibrillin-1 (FBN1) | |||||
| Gene Name | FBN1 | |||||
| Gene ID | 2200 | |||||
| Synonyms |
FBN; Fibrillin-1 [Cleaved into: Asprosin] |
|||||
| 3D Structure | ||||||
| Sequence |
MRRGRLLEIALGFTVLLASYTSHGADANLEAGNVKETRASRAKRRGGGGHDALKGPNVCG
SRYNAYCCPGWKTLPGGNQCIVPICRHSCGDGFCSRPNMCTCPSGQIAPSCGSRSIQHCN IRCMNGGSCSDDHCLCQKGYIGTHCGQPVCESGCLNGGRCVAPNRCACTYGFTGPQCERD YRTGPCFTVISNQMCQGQLSGIVCTKTLCCATVGRAWGHPCEMCPAQPHPCRRGFIPNIR TGACQDVDECQAIPGLCQGGNCINTVGSFECKCPAGHKLNEVSQKCEDIDECSTIPGICE GGECTNTVSSYFCKCPPGFYTSPDGTRCIDVRPGYCYTALTNGRCSNQLPQSITKMQCCC DAGRCWSPGVTVAPEMCPIRATEDFNKLCSVPMVIPGRPEYPPPPLGPIPPVLPVPPGFP PGPQIPVPRPPVEYLYPSREPPRVLPVNVTDYCQLVRYLCQNGRCIPTPGSYRCECNKGF QLDLRGECIDVDECEKNPCAGGECINNQGSYTCQCRAGYQSTLTRTECRDIDECLQNGRI CNNGRCINTDGSFHCVCNAGFHVTRDGKNCEDMDECSIRNMCLNGMCINEDGSFKCICKP GFQLASDGRYCKDINECETPGICMNGRCVNTDGSYRCECFPGLAVGLDGRVCVDTHMRST CYGGYKRGQCIKPLFGAVTKSECCCASTEYAFGEPCQPCPAQNSAEYQALCSSGPGMTSA GSDINECALDPDICPNGICENLRGTYKCICNSGYEVDSTGKNCVDINECVLNSLLCDNGQ CRNTPGSFVCTCPKGFIYKPDLKTCEDIDECESSPCINGVCKNSPGSFICECSSESTLDP TKTICIETIKGTCWQTVIDGRCEININGATLKSQCCSSLGAAWGSPCTLCQVDPICGKGY SRIKGTQCEDIDECEVFPGVCKNGLCVNTRGSFKCQCPSGMTLDATGRICLDIRLETCFL RYEDEECTLPIAGRHRMDACCCSVGAAWGTEECEECPMRNTPEYEELCPRGPGFATKEIT NGKPFFKDINECKMIPSLCTHGKCRNTIGSFKCRCDSGFALDSEERNCTDIDECRISPDL CGRGQCVNTPGDFECKCDEGYESGFMMMKNCMDIDECQRDPLLCRGGVCHNTEGSYRCEC PPGHQLSPNISACIDINECELSAHLCPNGRCVNLIGKYQCACNPGYHSTPDRLFCVDIDE CSIMNGGCETFCTNSEGSYECSCQPGFALMPDQRSCTDIDECEDNPNICDGGQCTNIPGE YRCLCYDGFMASEDMKTCVDVNECDLNPNICLSGTCENTKGSFICHCDMGYSGKKGKTGC TDINECEIGAHNCGKHAVCTNTAGSFKCSCSPGWIGDGIKCTDLDECSNGTHMCSQHADC KNTMGSYRCLCKEGYTGDGFTCTDLDECSENLNLCGNGQCLNAPGGYRCECDMGFVPSAD GKACEDIDECSLPNICVFGTCHNLPGLFRCECEIGYELDRSGGNCTDVNECLDPTTCISG NCVNTPGSYICDCPPDFELNPTRVGCVDTRSGNCYLDIRPRGDNGDTACSNEIGVGVSKA SCCCSLGKAWGTPCEMCPAVNTSEYKILCPGGEGFRPNPITVILEDIDECQELPGLCQGG KCINTFGSFQCRCPTGYYLNEDTRVCDDVNECETPGICGPGTCYNTVGNYTCICPPDYMQ VNGGNNCMDMRRSLCYRNYYADNQTCDGELLFNMTKKMCCCSYNIGRAWNKPCEQCPIPS TDEFATLCGSQRPGFVIDIYTGLPVDIDECREIPGVCENGVCINMVGSFRCECPVGFFYN DKLLVCEDIDECQNGPVCQRNAECINTAGSYRCDCKPGYRFTSTGQCNDRNECQEIPNIC SHGQCIDTVGSFYCLCHTGFKTNDDQTMCLDINECERDACGNGTCRNTIGSFNCRCNHGF ILSHNNDCIDVDECASGNGNLCRNGQCINTVGSFQCQCNEGYEVAPDGRTCVDINECLLE PRKCAPGTCQNLDGSYRCICPPGYSLQNEKCEDIDECVEEPEICALGTCSNTEGSFKCLC PEGFSLSSSGRRCQDLRMSYCYAKFEGGKCSSPKSRNHSKQECCCALKGEGWGDPCELCP TEPDEAFRQICPYGSGIIVGPDDSAVDMDECKEPDVCKHGQCINTDGSYRCECPFGYILA GNECVDTDECSVGNPCGNGTCKNVIGGFECTCEEGFEPGPMMTCEDINECAQNPLLCAFR CVNTYGSYECKCPVGYVLREDRRMCKDEDECEEGKHDCTEKQMECKNLIGTYMCICGPGY QRRPDGEGCVDENECQTKPGICENGRCLNTRGSYTCECNDGFTASPNQDECLDNREGYCF TEVLQNMCQIGSSNRNPVTKSECCCDGGRGWGPHCEICPFQGTVAFKKLCPHGRGFMTNG ADIDECKVIHDVCRNGECVNDRGSYHCICKTGYTPDITGTSCVDLNECNQAPKPCNFICK NTEGSYQCSCPKGYILQEDGRSCKDLDECATKQHNCQFLCVNTIGGFTCKCPPGFTQHHT SCIDNNECTSDINLCGSKGICQNTPGSFTCECQRGFSLDQTGSSCEDVDECEGNHRCQHG CQNIIGGYRCSCPQGYLQHYQWNQCVDENECLSAHICGGASCHNTLGSYKCMCPAGFQYE QFSGGCQDINECGSAQAPCSYGCSNTEGGYLCGCPPGYFRIGQGHCVSGMGMGRGNPEPP VSGEMDDNSLSPEACYECKINGYPKRGRKRRSTNETDASNIEDQSETEANVSLASWDVEK TAIFAFNISHVSNKVRILELLPALTTLTNHNRYLIESGNEDGFFKINQKEGISYLHFTKK KPVAGTYSLQISSTPLYKKKELNQLEDKYDKDYLSGELGDNLKMKIQVLLH |
|||||
| Target Bioclass |
Other
|
|||||
| Family |
Fibrillin family
|
|||||
| Subcellular location |
Secreted; Secreted, extracellular space, extracellular matrix
|
|||||
| Function |
[Fibrillin-1]: Structural component of the 10-12 nm diameter microfibrils of the extracellular matrix, which conveys both structural and regulatory properties to load-bearing connective tissues. Fibrillin-1-containing microfibrils provide long-term force bearing structural support. In tissues such as the lung, blood vessels and skin, microfibrils form the periphery of the elastic fiber, acting as a scaffold for the deposition of elastin. In addition, microfibrils can occur as elastin-independent networks in tissues such as the ciliary zonule, tendon, cornea and glomerulus where they provide tensile strength and have anchoring roles. Fibrillin-1 also plays a key role in tissue homeostasis through specific interactions with growth factors, such as the bone morphogenetic proteins (BMPs), growth and differentiation factors (GDFs) and latent transforming growth factor-beta-binding proteins (LTBPs), cell-surface integrins and other extracellular matrix protein and proteoglycan components. Regulates osteoblast maturation by controlling TGF-beta bioavailability and calibrating TGF-beta and BMP levels, respectively. Negatively regulates osteoclastogenesis by binding and sequestering an osteoclast differentiation and activation factor TNFSF11. This leads to disruption of TNFSF11-induced Ca(2+) signaling and impairment of TNFSF11-mediated nuclear translocation and activation of transcription factor NFATC1 which regulates genes important for osteoclast differentiation and function. Mediates cell adhesion via its binding to cell surface receptors integrins ITGAV:ITGB3 and ITGA5:ITGB1. Binds heparin and this interaction has an important role in the assembly of microfibrils.; [Asprosin]: Adipokine secreted by white adipose tissue that plays an important regulatory role in the glucose metabolism of liver, muscle and pancreas. Hormone that targets the liver in response to fasting to increase plasma glucose levels. Binds the olfactory receptor OR4M1 at the surface of hepatocytes and promotes hepatocyte glucose release by activating the protein kinase A activity in the liver, resulting in rapid glucose release into the circulation. May act as a regulator of adaptive thermogenesis by inhibiting browning and energy consumption, while increasing lipid deposition in white adipose tissue. Also acts as an orexigenic hormone that increases appetite: crosses the blood brain barrier and exerts effects on the hypothalamus. In the arcuate nucleus of the hypothalamus, asprosin directly activates orexigenic AgRP neurons and indirectly inhibits anorexigenic POMC neurons, resulting in appetite stimulation. Activates orexigenic AgRP neurons via binding to the olfactory receptor OR4M1. May also play a role in sperm motility in testis via interaction with OR4M1 receptor.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
IPM Probe Info |
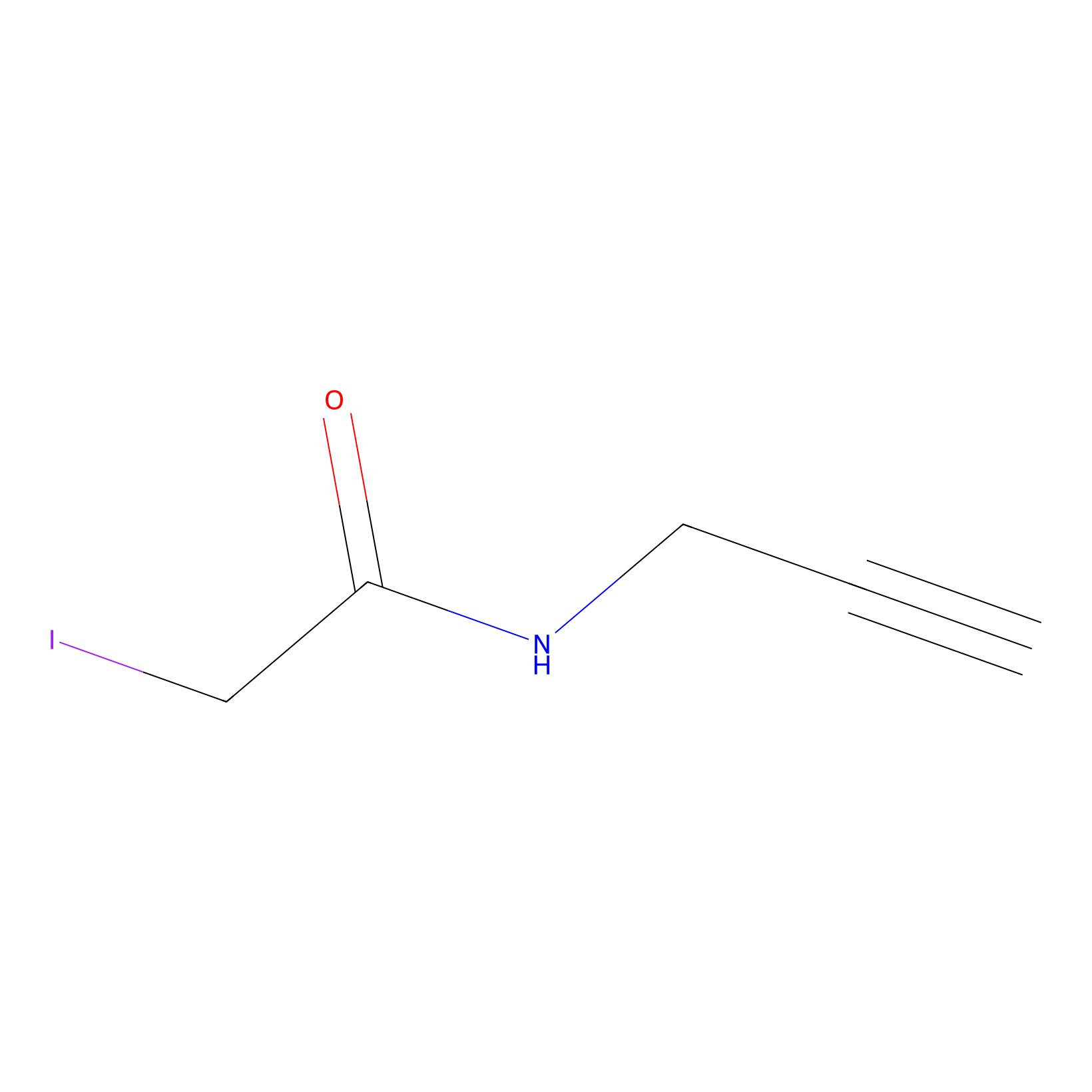 |
C950(7.21) | LDD2229 | [1] | |
|
DBIA Probe Info |
 |
C617(1.12); C623(1.12); C59(1.12); C1971(1.11) | LDD0078 | [2] | |
|
IA-alkyne Probe Info |
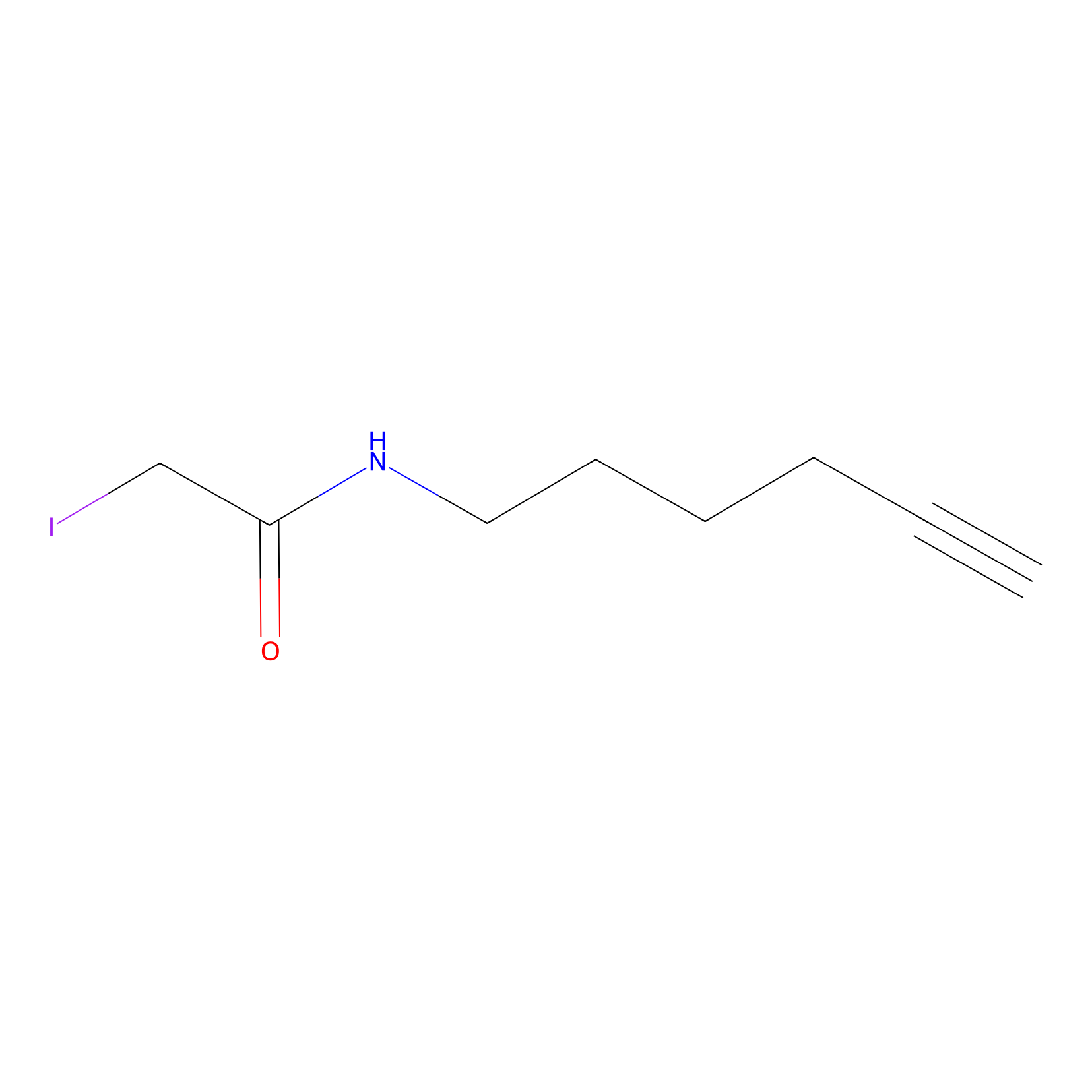 |
N.A. | LDD0165 | [3] | |
|
TFBX Probe Info |
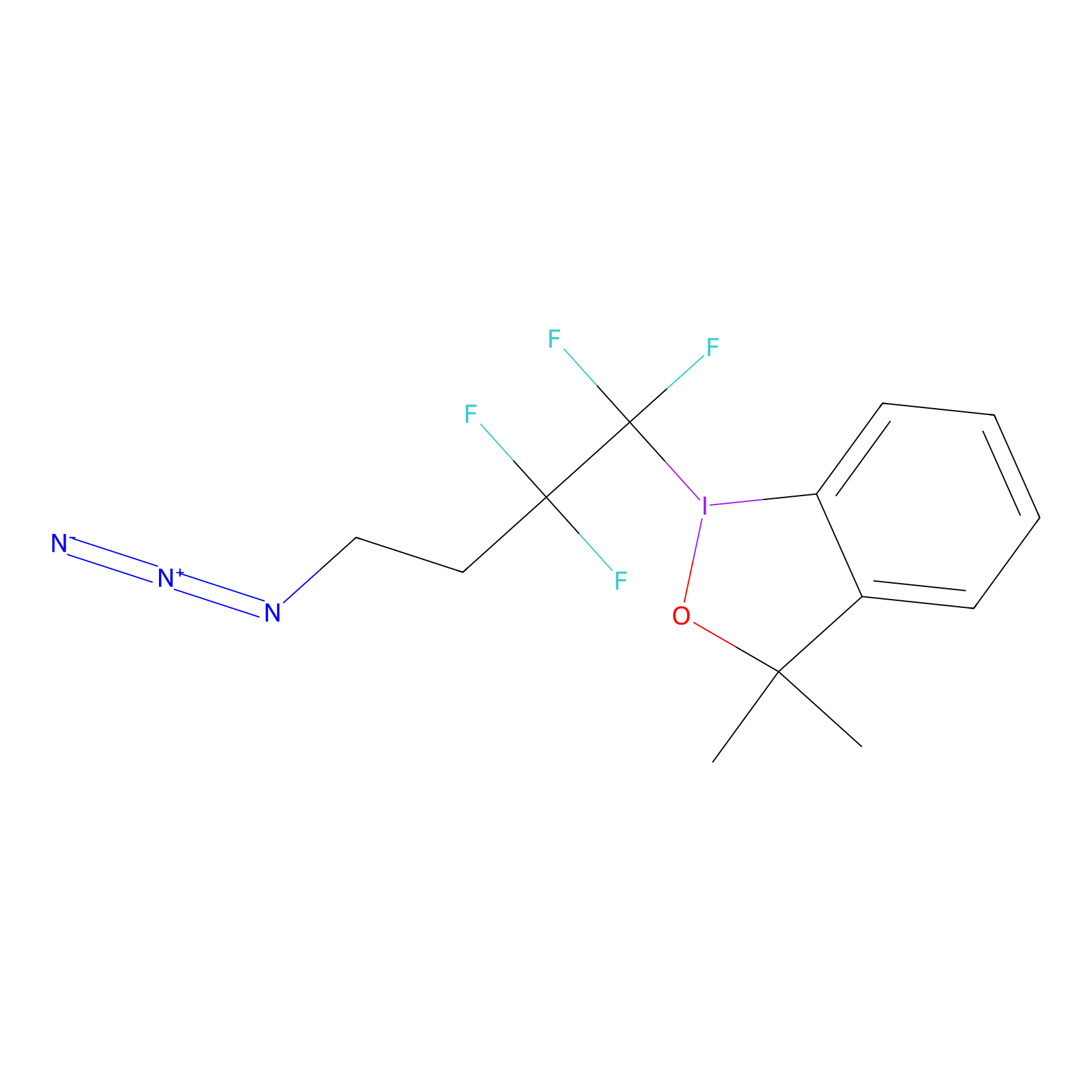 |
N.A. | LDD0148 | [4] | |
|
Acrolein Probe Info |
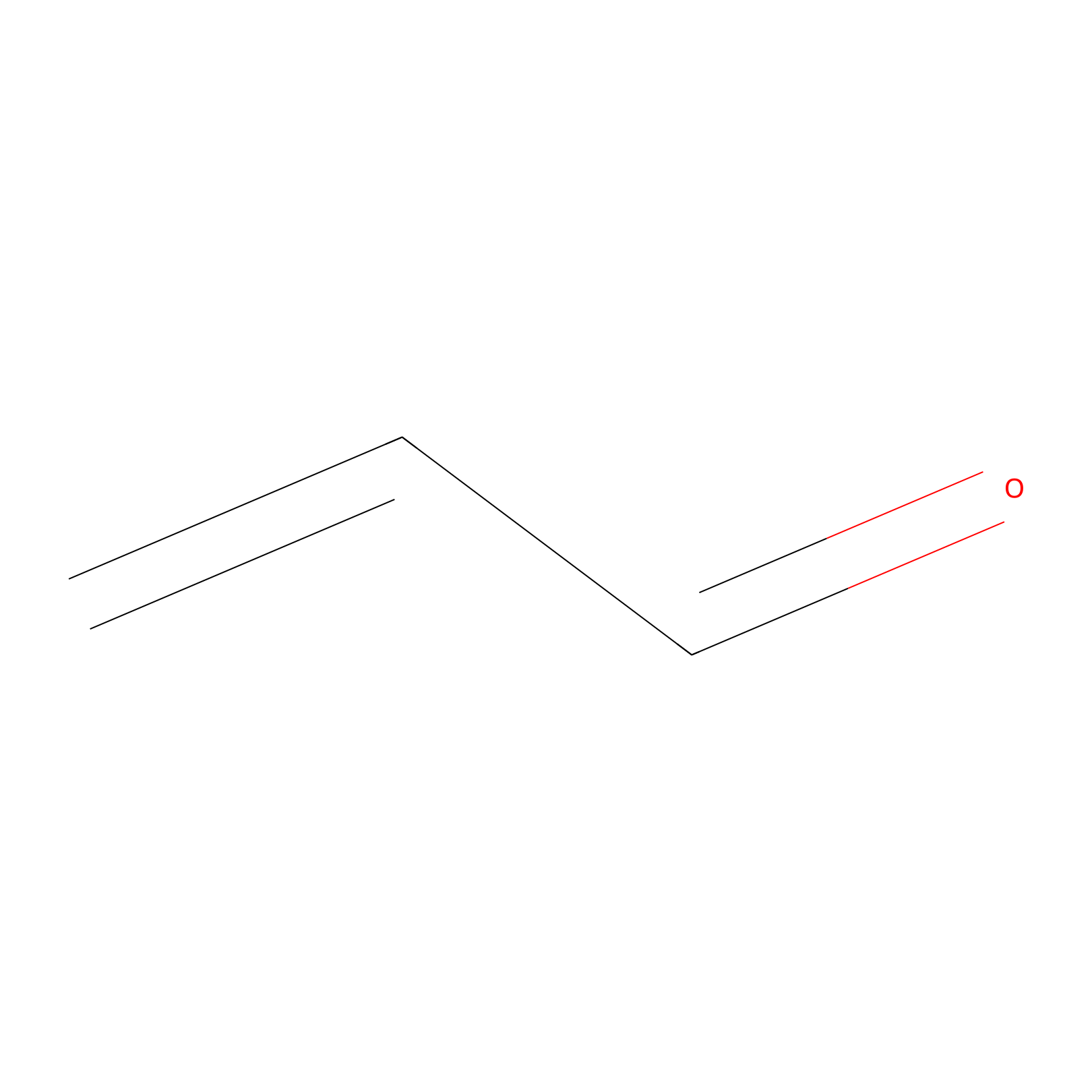 |
N.A. | LDD0217 | [5] | |
|
Methacrolein Probe Info |
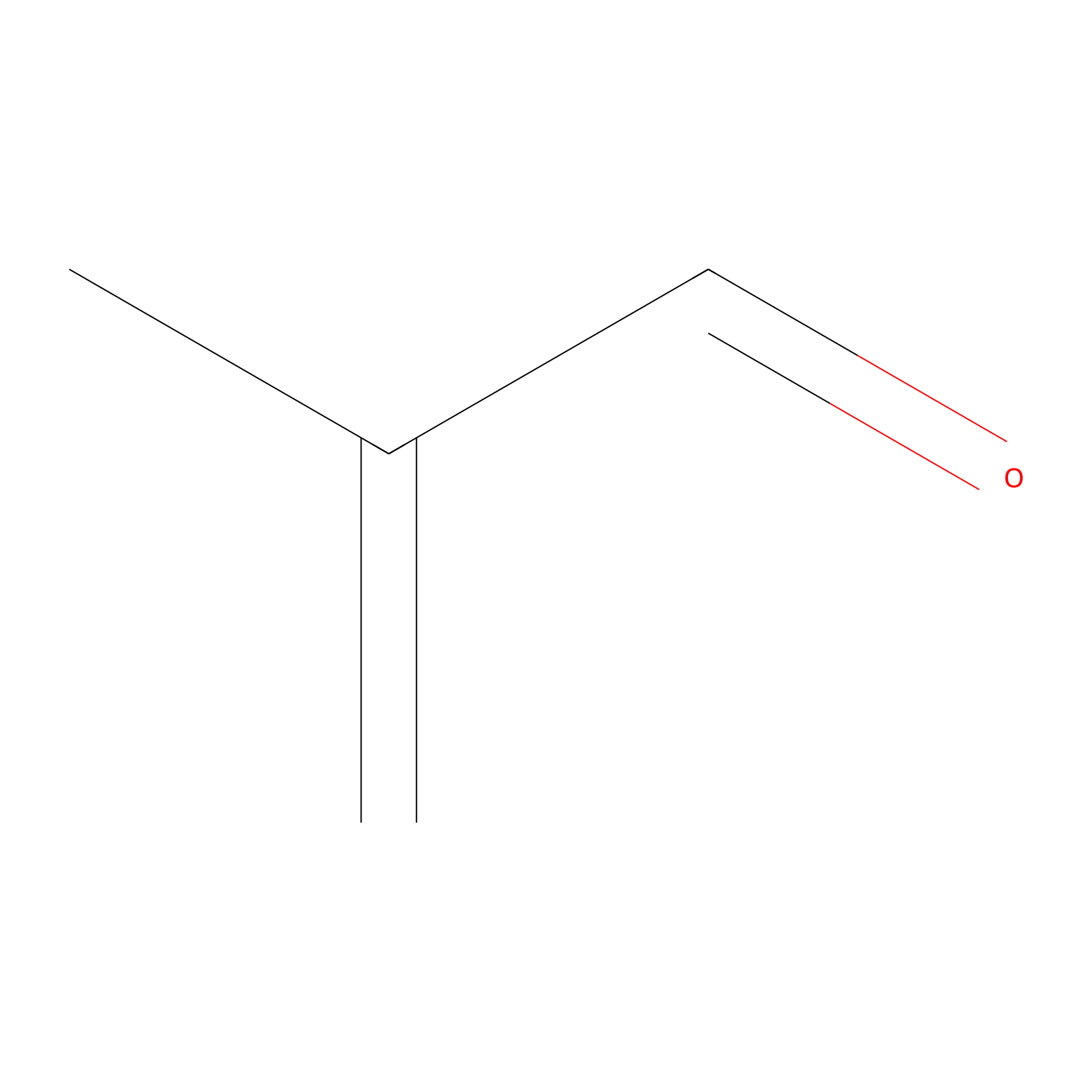 |
C967(0.00); C1039(0.00); C1562(0.00); C80(0.00) | LDD0218 | [5] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0020 | ARS-1620 | HCC44 | C617(1.12); C623(1.12); C59(1.12); C1971(1.11) | LDD0078 | [2] |
| LDCM0108 | Chloroacetamide | HeLa | N.A. | LDD0222 | [5] |
| LDCM0022 | KB02 | 42-MG-BA | C2232(1.03); C2686(1.33); C958(1.82); C1008(1.25) | LDD2244 | [6] |
| LDCM0023 | KB03 | 42-MG-BA | C2686(1.08); C958(1.53) | LDD2661 | [6] |
| LDCM0024 | KB05 | NCI-H3122 | C2258(1.35) | LDD3360 | [6] |
| LDCM0109 | NEM | HeLa | N.A. | LDD0227 | [5] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Enzyme
| Protein name | Family | Uniprot ID | |||
|---|---|---|---|---|---|
| Protein-lysine 6-oxidase (LOX) | Lysyl oxidase family | P28300 | |||
Other
References
