Details of the Target
General Information of Target
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
AZ-9 Probe Info |
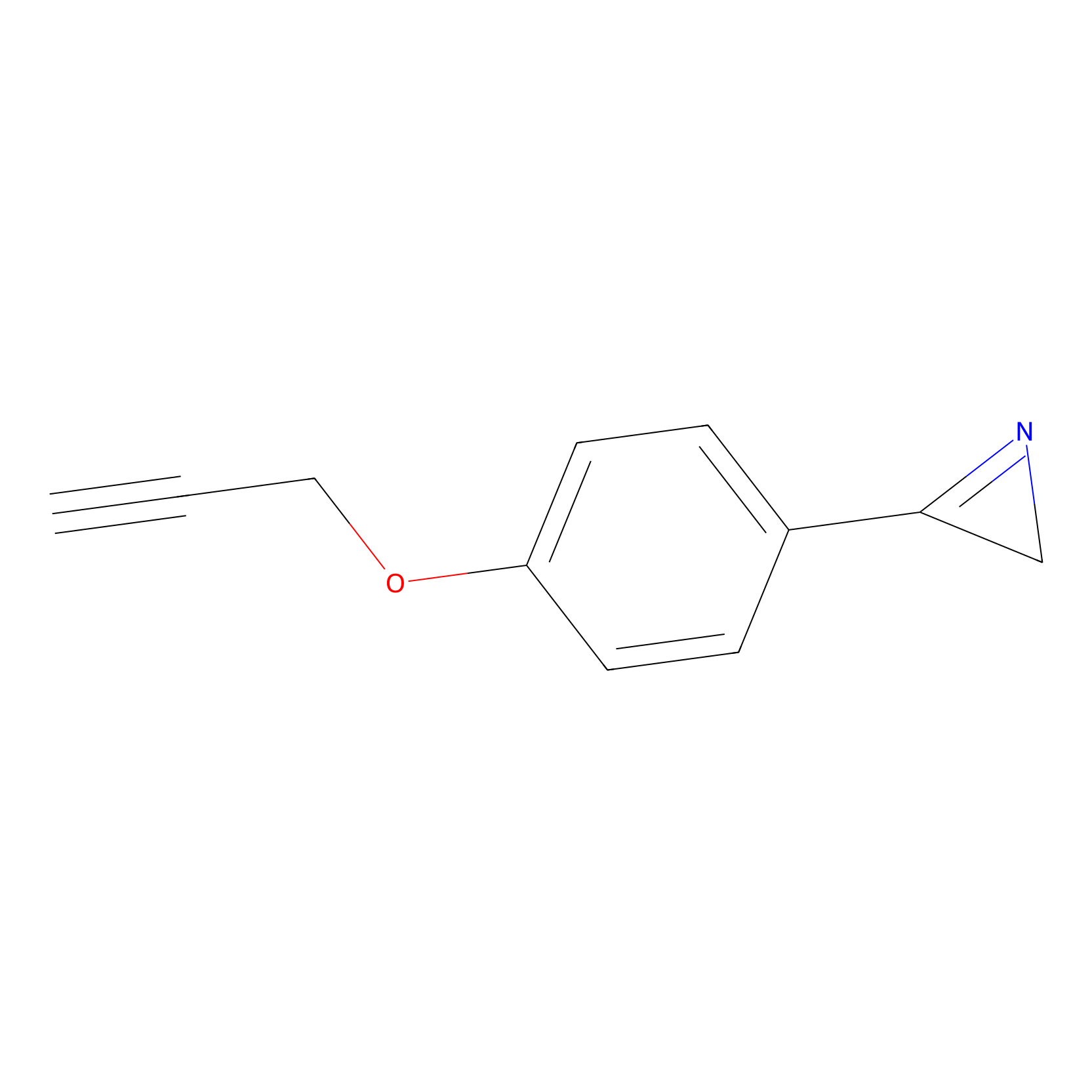 |
E210(0.90) | LDD2208 | [1] | |
|
DBIA Probe Info |
 |
C344(1.06) | LDD3321 | [2] | |
|
BTD Probe Info |
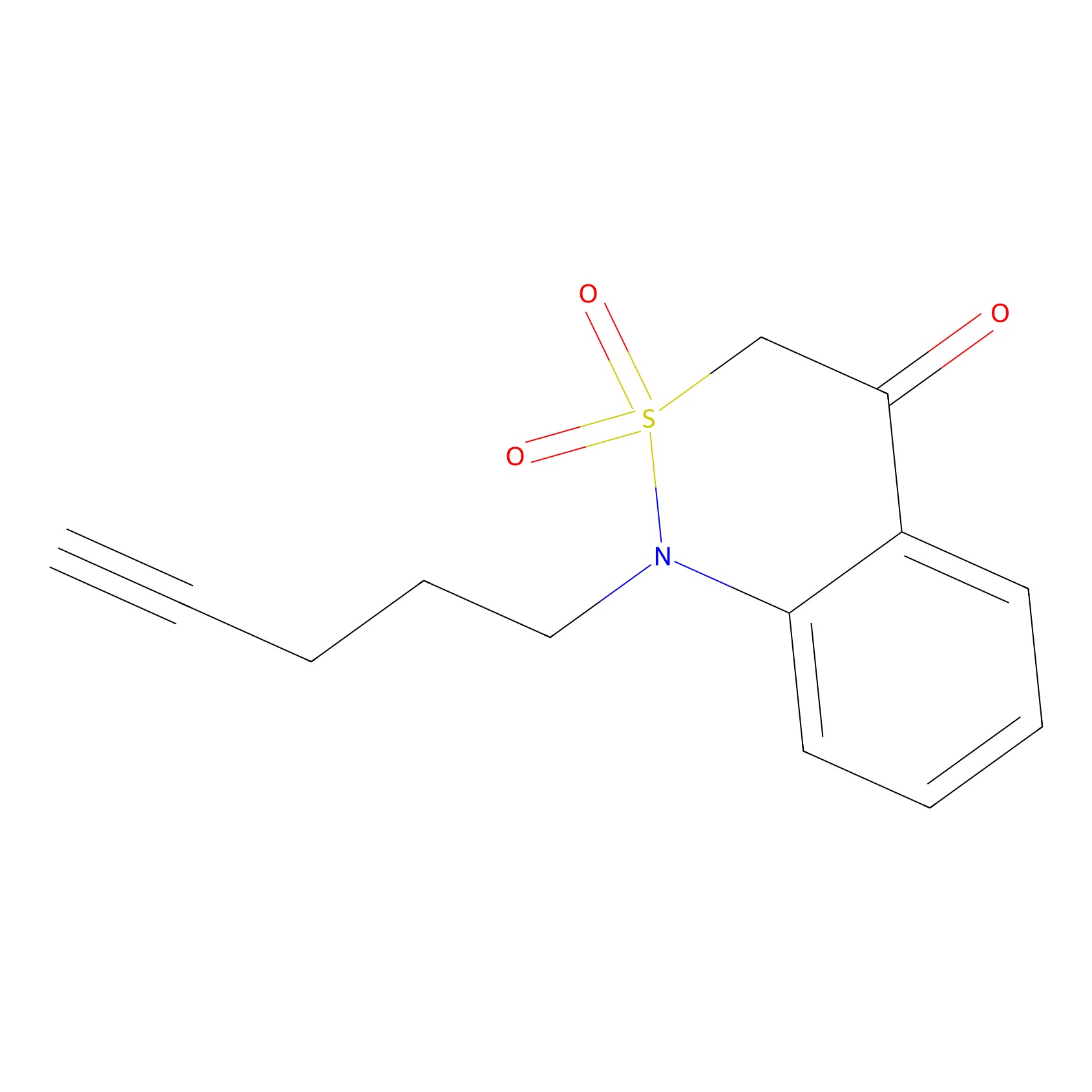 |
C217(1.80) | LDD1700 | [3] | |
|
IA-alkyne Probe Info |
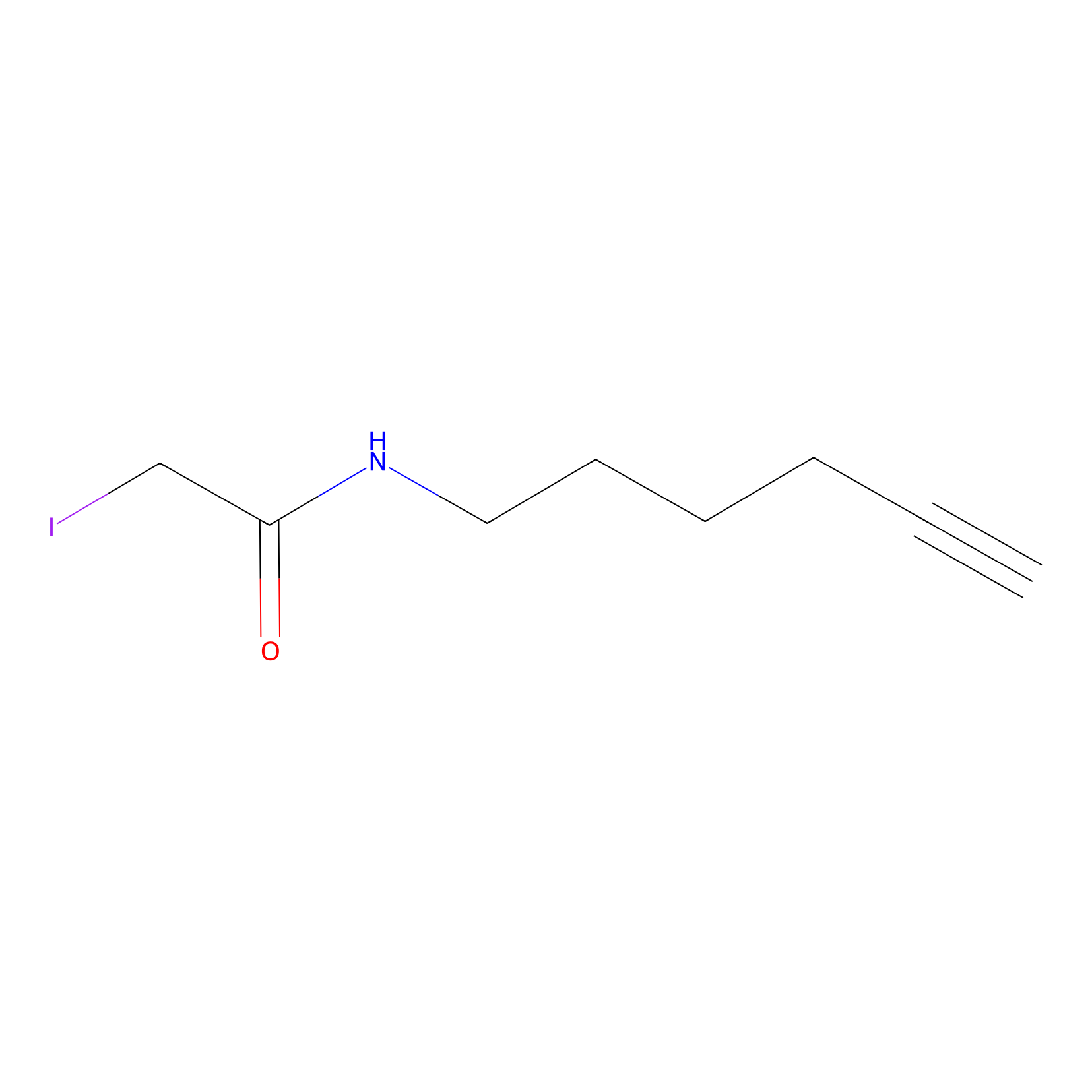 |
C244(0.00); C428(0.00); C217(0.00); C344(0.00) | LDD0162 | [4] | |
|
JW-RF-010 Probe Info |
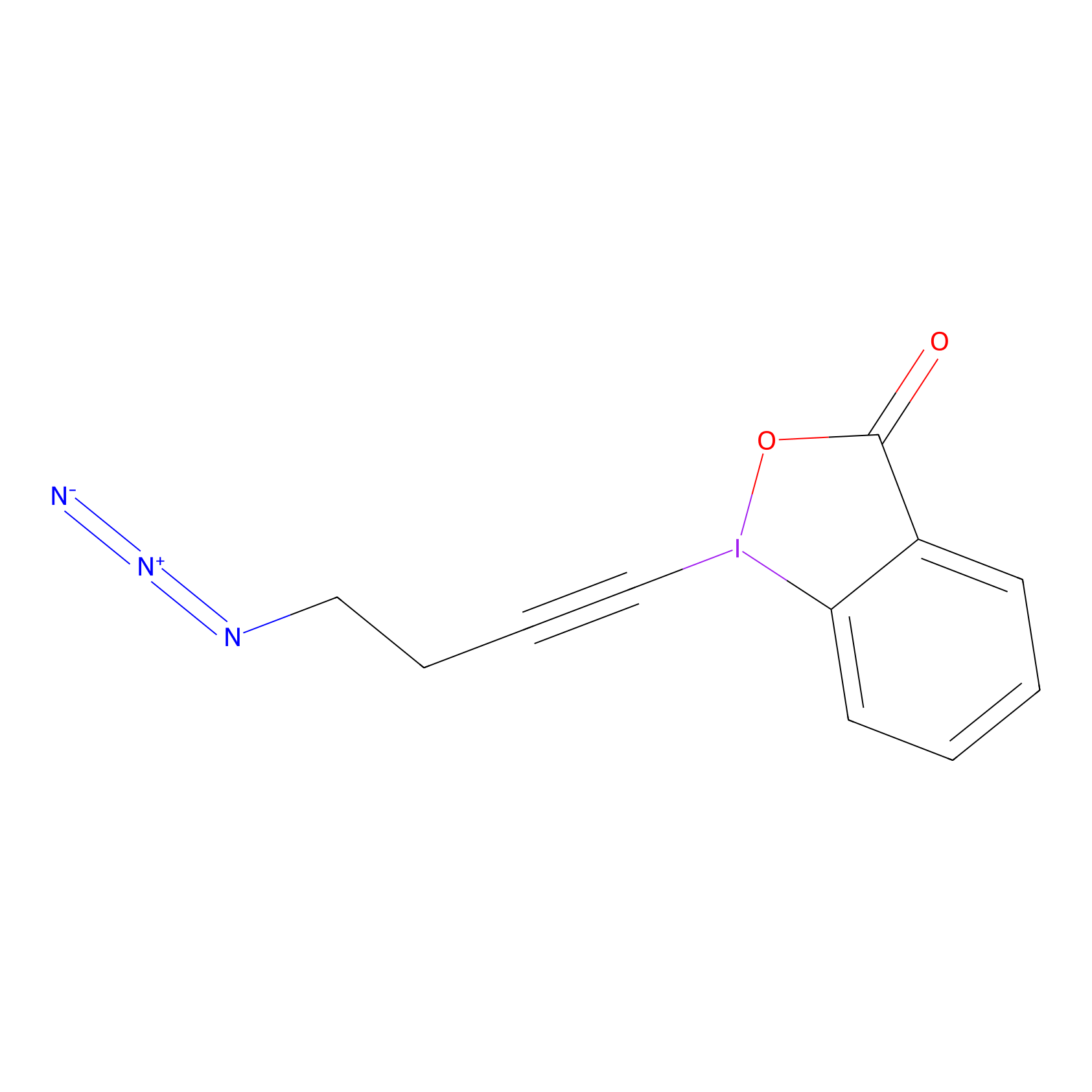 |
N.A. | LDD0026 | [5] | |
|
WYneN Probe Info |
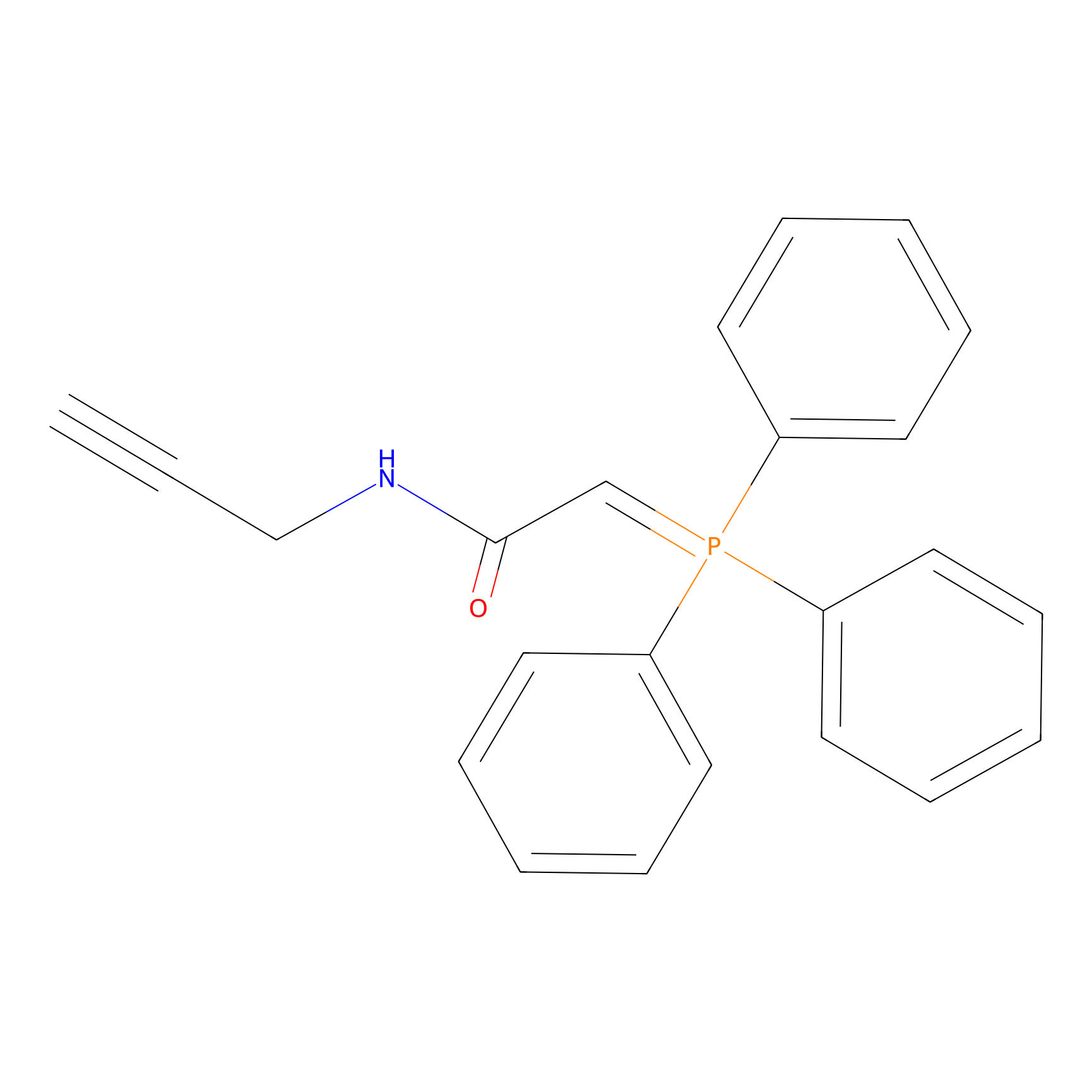 |
C217(0.00); C428(0.00); C223(0.00); C244(0.00) | LDD0021 | [6] | |
|
WYneO Probe Info |
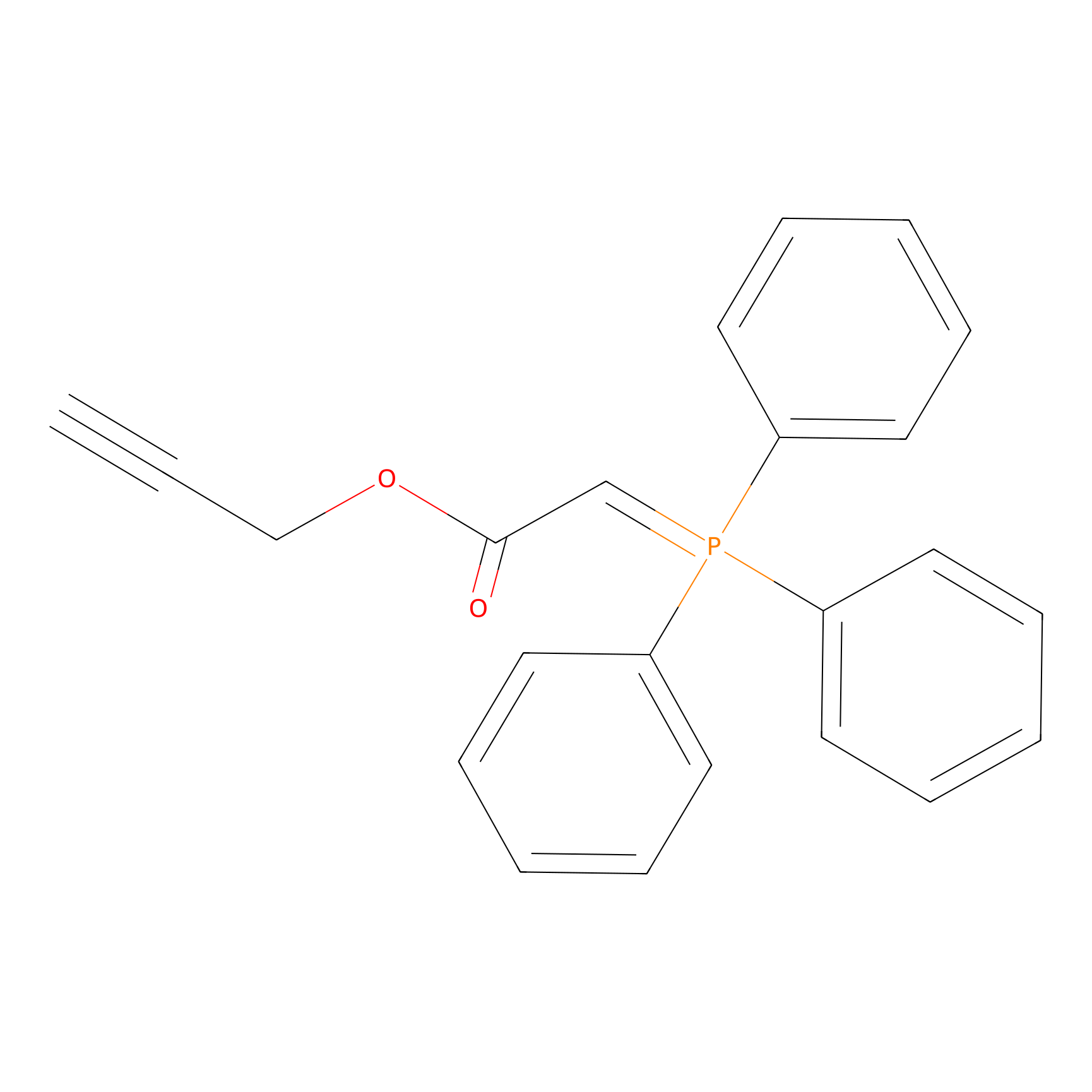 |
C428(0.00); C244(0.00); C217(0.00); C229(0.00) | LDD0022 | [6] | |
|
Acrolein Probe Info |
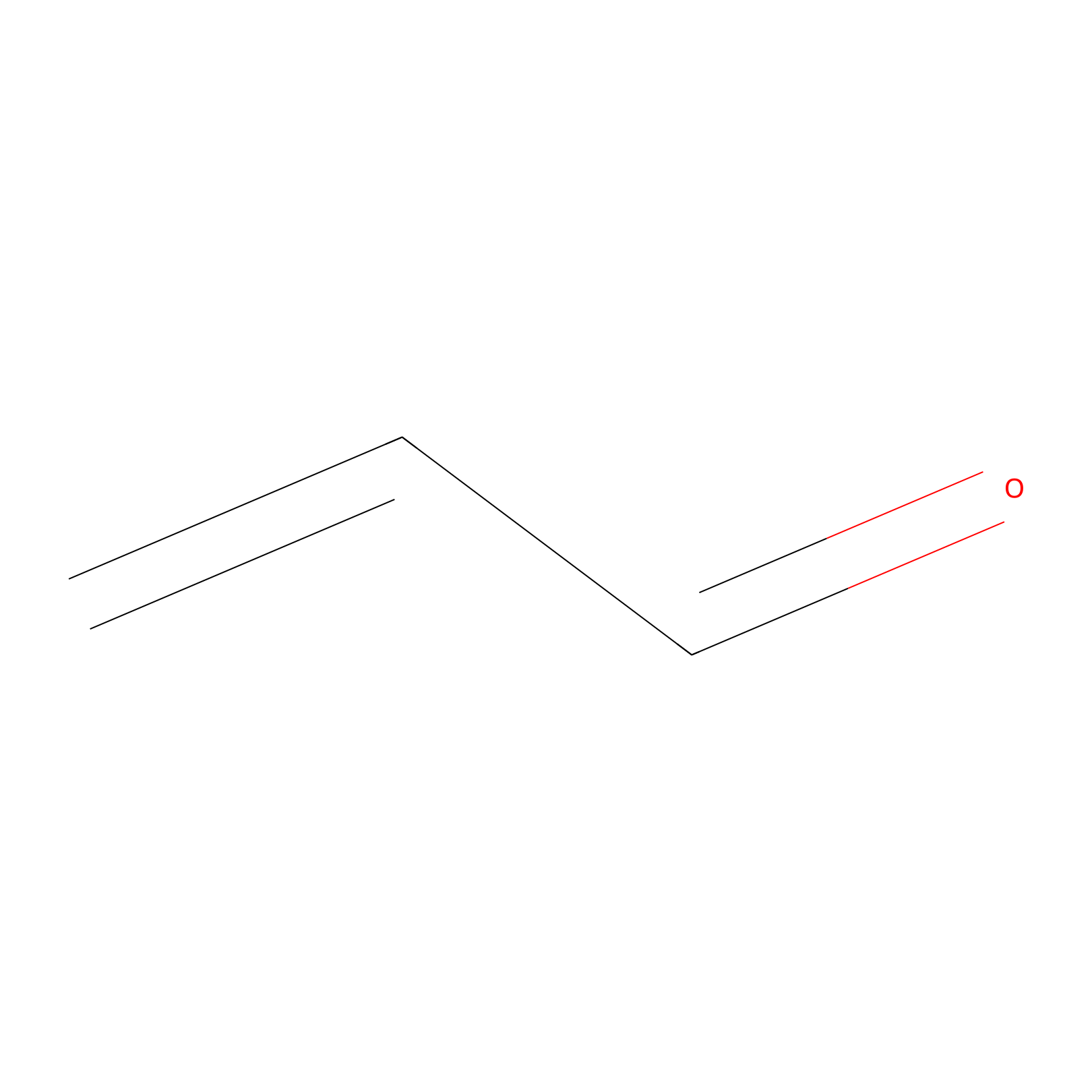 |
H203(0.00); C244(0.00); C229(0.00) | LDD0217 | [7] | |
|
Crotonaldehyde Probe Info |
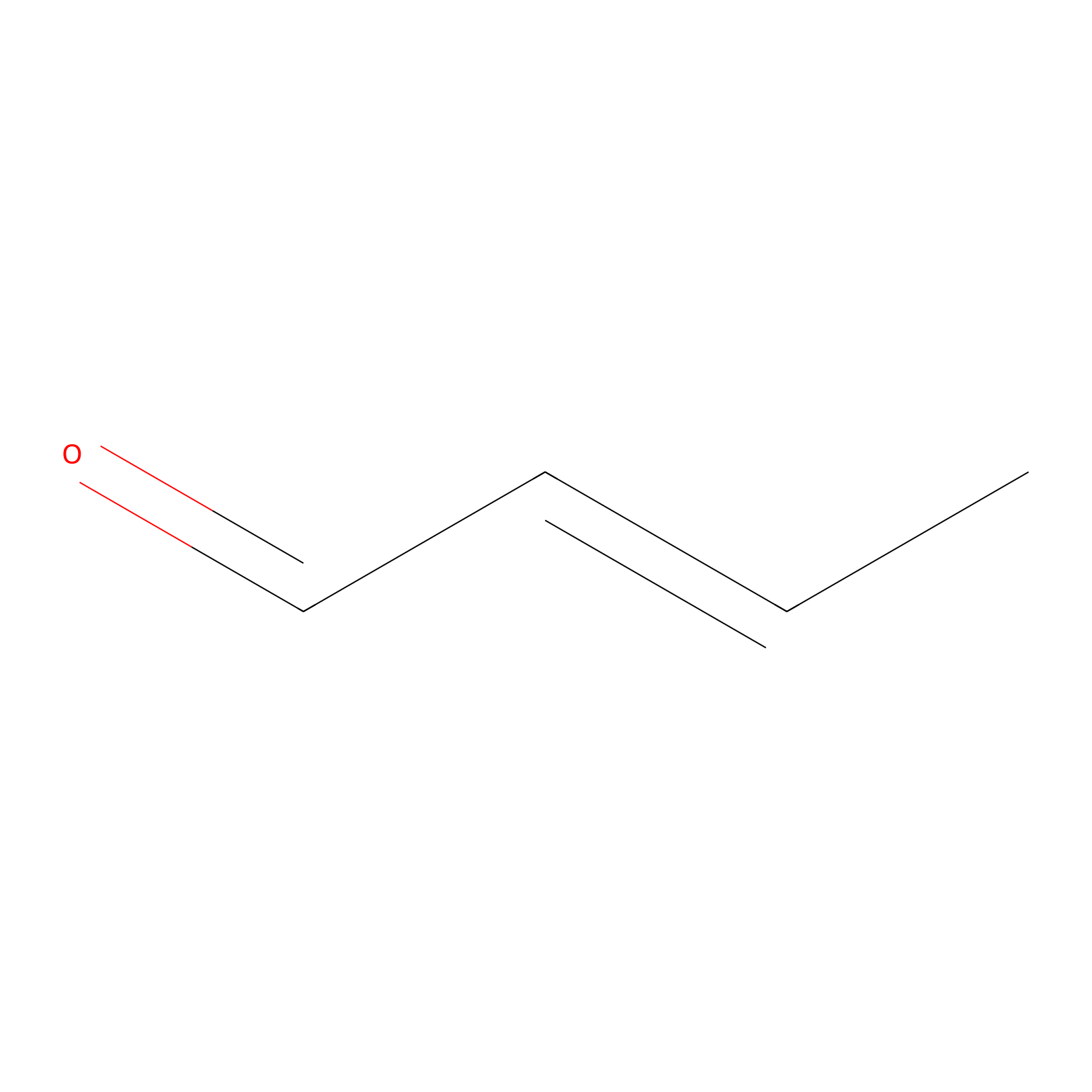 |
N.A. | LDD0219 | [7] | |
|
HHS-475 Probe Info |
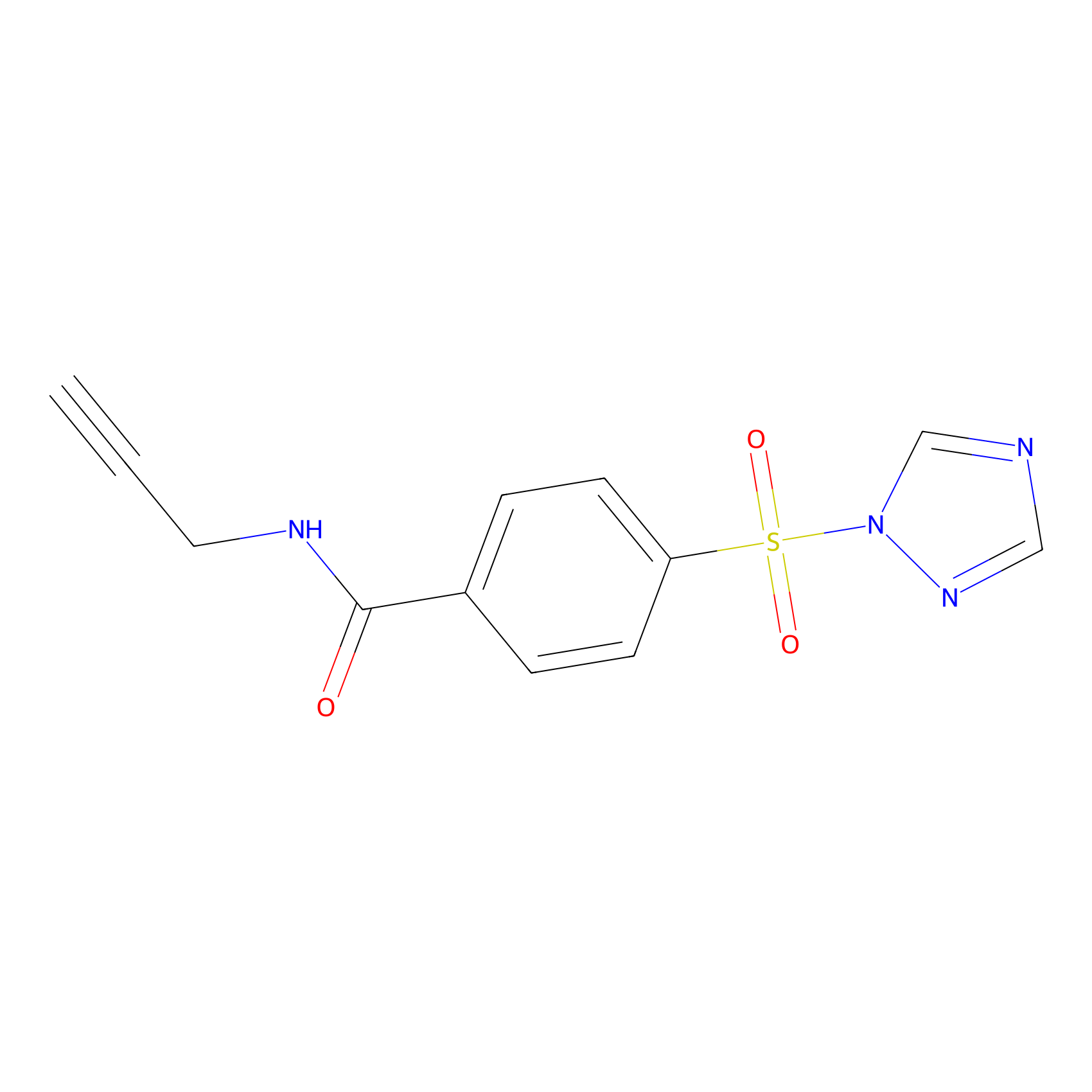 |
Y305(1.32) | LDD2238 | [8] | |
|
HHS-482 Probe Info |
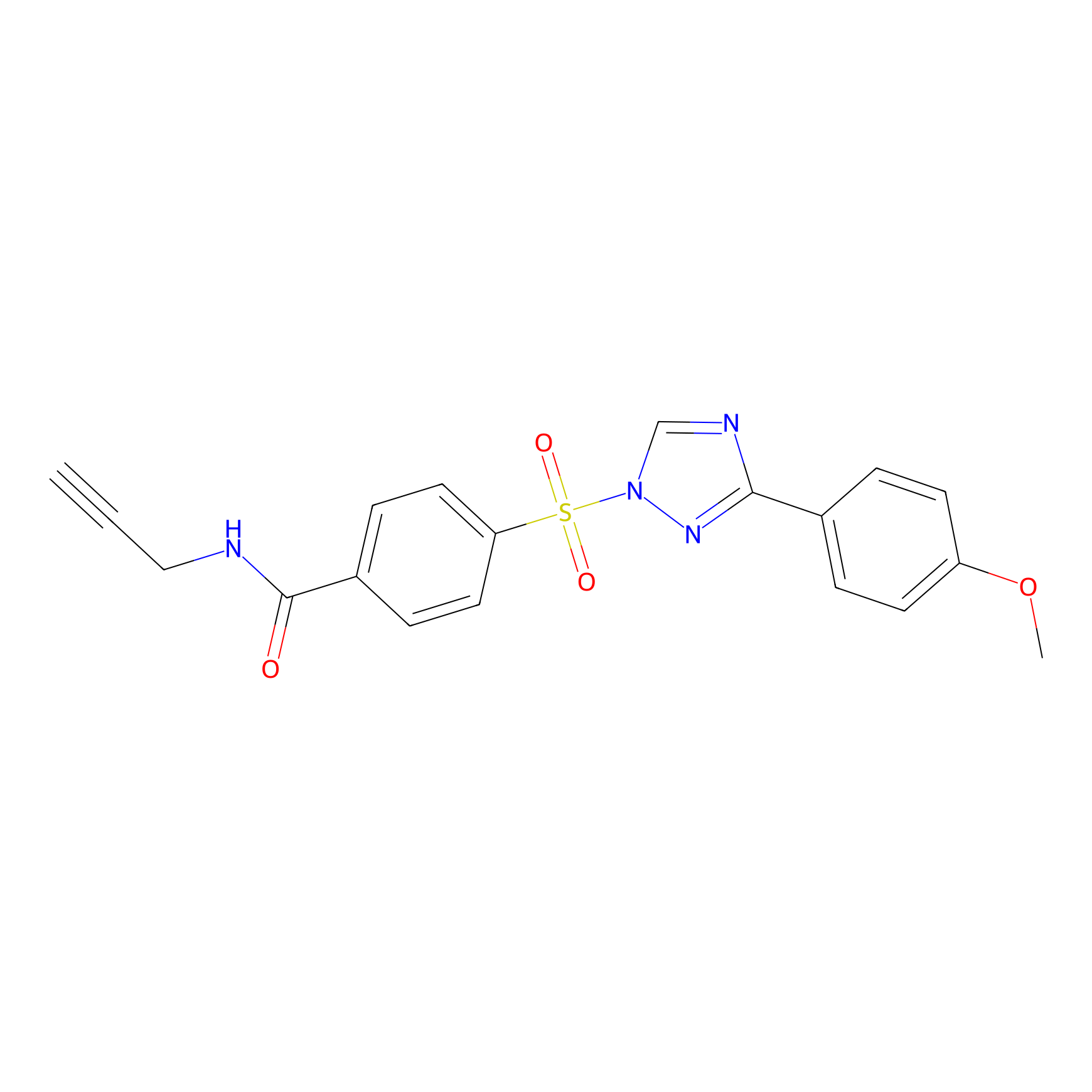 |
Y186(0.85); Y315(1.13) | LDD2239 | [8] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0524 | 2-Cyano-N-(2-morpholin-4-yl-ethyl)-acetamide | MDA-MB-231 | C217(0.60) | LDD2117 | [3] |
| LDCM0558 | 2-Cyano-N-phenylacetamide | MDA-MB-231 | C217(1.02) | LDD2152 | [3] |
| LDCM0108 | Chloroacetamide | HeLa | H53(0.00); H203(0.00); C229(0.00) | LDD0222 | [7] |
| LDCM0107 | IAA | HeLa | H203(0.00); H53(0.00); C229(0.00) | LDD0221 | [7] |
| LDCM0022 | KB02 | A-172 | C344(0.94) | LDD2251 | [2] |
| LDCM0023 | KB03 | A-172 | C344(1.31) | LDD2668 | [2] |
| LDCM0024 | KB05 | SH4 | C344(1.06) | LDD3321 | [2] |
| LDCM0109 | NEM | HeLa | H203(0.00); H53(0.00) | LDD0223 | [7] |
| LDCM0500 | Nucleophilic fragment 13a | MDA-MB-231 | C217(1.07) | LDD2093 | [3] |
| LDCM0514 | Nucleophilic fragment 20a | MDA-MB-231 | C217(0.98) | LDD2107 | [3] |
| LDCM0530 | Nucleophilic fragment 28a | MDA-MB-231 | C217(0.87) | LDD2123 | [3] |
| LDCM0543 | Nucleophilic fragment 38 | MDA-MB-231 | C217(1.08) | LDD2136 | [3] |
| LDCM0211 | Nucleophilic fragment 3b | MDA-MB-231 | C217(1.80) | LDD1700 | [3] |
| LDCM0550 | Nucleophilic fragment 5a | MDA-MB-231 | C217(3.53) | LDD2144 | [3] |
| LDCM0552 | Nucleophilic fragment 6a | MDA-MB-231 | C217(1.20) | LDD2146 | [3] |
The Interaction Atlas With This Target
References
