Details of the Target
General Information of Target
| Target ID | LDTP00608 | |||||
|---|---|---|---|---|---|---|
| Target Name | DNA-directed RNA polymerase III subunit RPC7 (POLR3G) | |||||
| Gene Name | POLR3G | |||||
| Gene ID | 10622 | |||||
| Synonyms |
DNA-directed RNA polymerase III subunit RPC7; RNA polymerase III subunit C7; DNA-directed RNA polymerase III subunit G; RNA polymerase III 32 kDa apha subunit; RPC32-alpha; RNA polymerase III 32 kDa subunit; RPC32
|
|||||
| 3D Structure | ||||||
| Sequence |
MAGNKGRGRAAYTFNIEAVGFSKGEKLPDVVLKPPPLFPDTDYKPVPLKTGEGEEYMLAL
KQELRETMKRMPYFIETPEERQDIERYSKRYMKVYKEEWIPDWRRLPREMMPRNKCKKAG PKPKKAKDAGKGTPLTNTEDVLKKMEELEKRGDGEKSDEENEEKEGSKEKSKEGDDDDDD DAAEQEEYDEEEQEEENDYINSYFEDGDDFGADSDDNMDEATY |
|||||
| Target Bioclass |
Enzyme
|
|||||
| Family |
Eukaryotic RPC7 RNA polymerase subunit family
|
|||||
| Subcellular location |
Nucleus
|
|||||
| Function |
DNA-dependent RNA polymerase catalyzes the transcription of DNA into RNA using the four ribonucleoside triphosphates as substrates. Specific peripheric component of RNA polymerase III (Pol III) which synthesizes small non-coding RNAs including 5S rRNA, snRNAs, tRNAs and miRNAs from at least 500 distinct genomic loci. Acts as a long tether that bridges POLR3C/RPC3-POLR3F/RPC6-POLR3G/RPC7 heterotrimer and the mobile stalk of Pol III, coordinating the dynamics of Pol III stalk and clamp modules during the transition from apo to elongation state. Pol III exists as two alternative complexes defined by the mutually exclusive incorporation of subunit POLR3G/RPC7alpha or POLR3GL/RPC7beta. POLR3G/RPC7alpha modulates Pol III transcriptome by specifically enhancing the transcription of snaR-A non-coding RNAs. At resting state, occupies the active site of apo Pol III and keeps Pol III in an autoinhibitory mode, preventing non-specific transcription. Pol III plays a key role in sensing and limiting infection by intracellular bacteria and DNA viruses. Acts as a nuclear and cytosolic DNA sensor involved in innate immune response. Can sense non-self dsDNA that serves as template for transcription into dsRNA. The non-self RNA polymerase III transcripts, such as Epstein-Barr virus-encoded RNAs (EBERs), induce type I interferon and NF-kappa-B through the RIG-I pathway.
|
|||||
| Uniprot ID | ||||||
| Ensemble ID | ||||||
| HGNC ID | ||||||
Probe(s) Labeling This Target
ABPP Probe
| Probe name | Structure | Binding Site(Ratio) | Interaction ID | Ref | |
|---|---|---|---|---|---|
|
m-APA Probe Info |
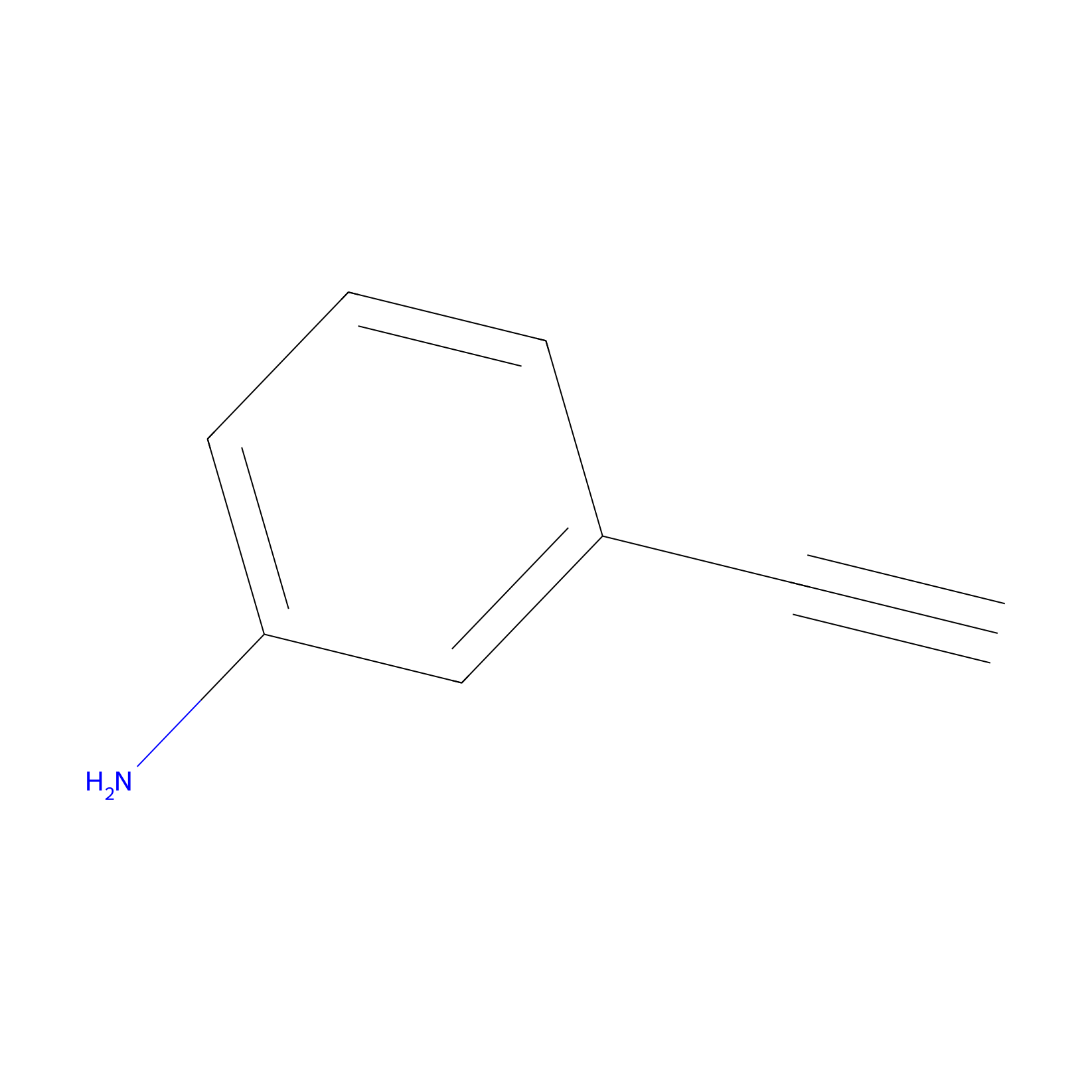 |
15.00 | LDD0402 | [1] | |
|
HHS-475 Probe Info |
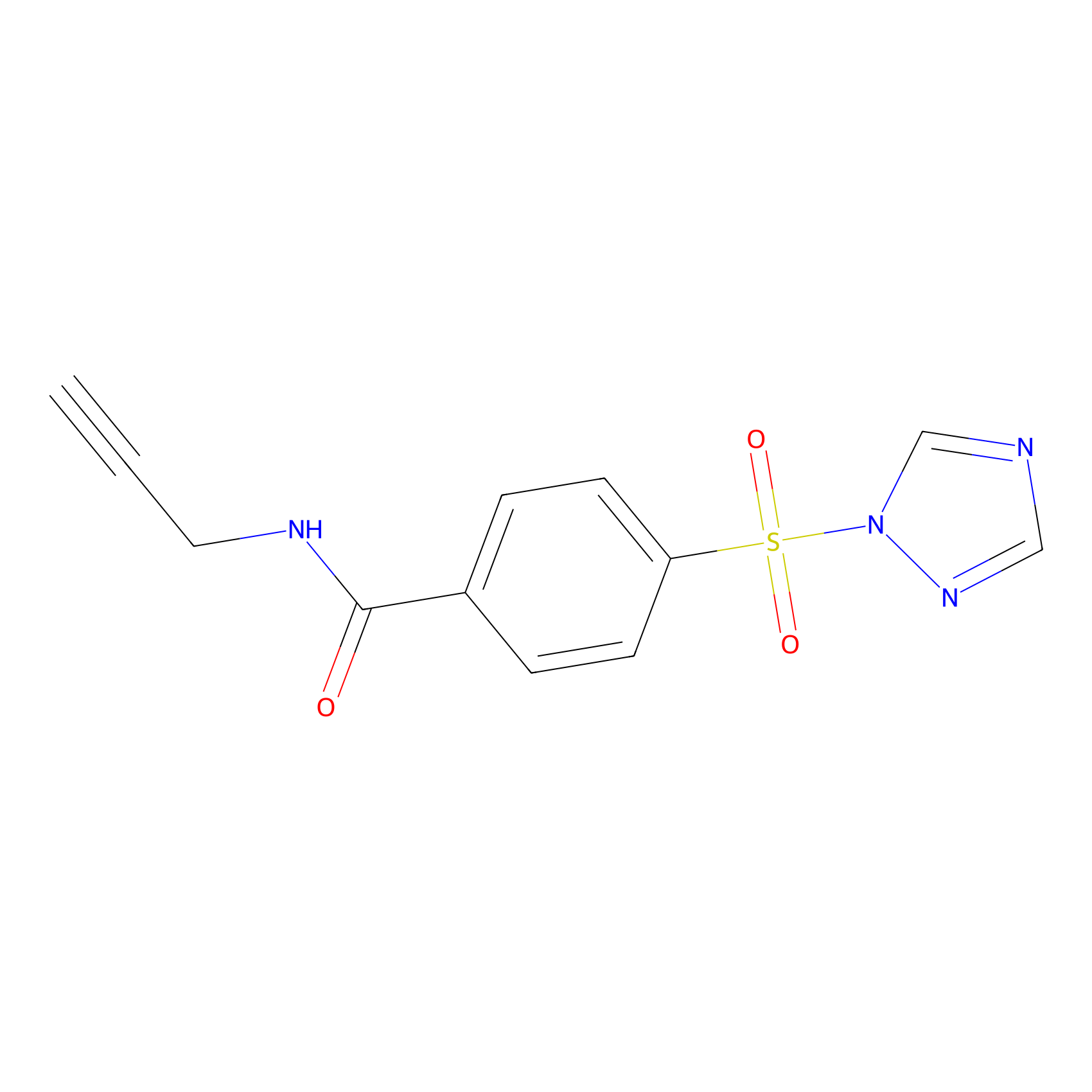 |
Y56(1.38); Y12(1.78) | LDD0264 | [2] | |
|
SF Probe Info |
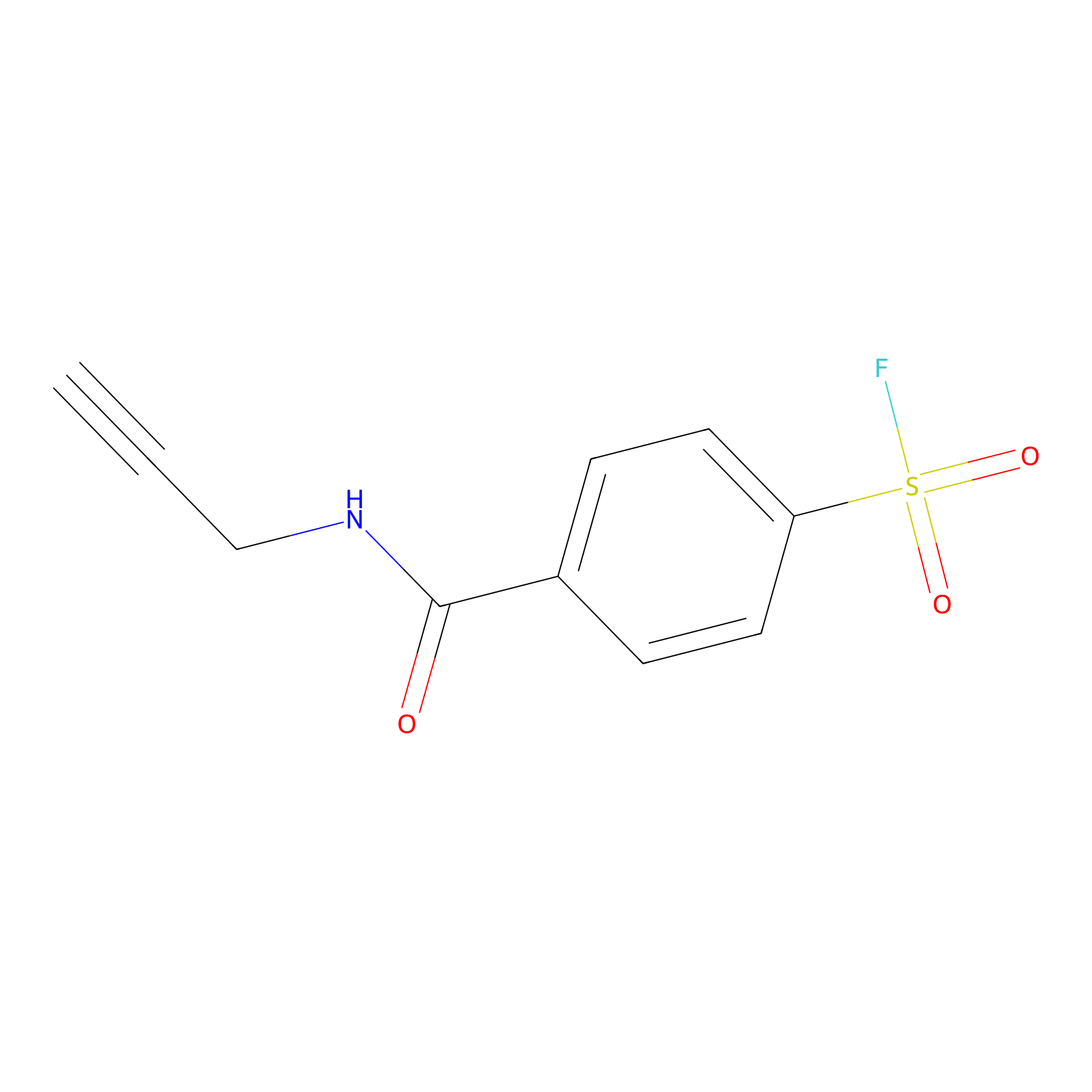 |
N.A. | LDD0028 | [3] | |
|
AOyne Probe Info |
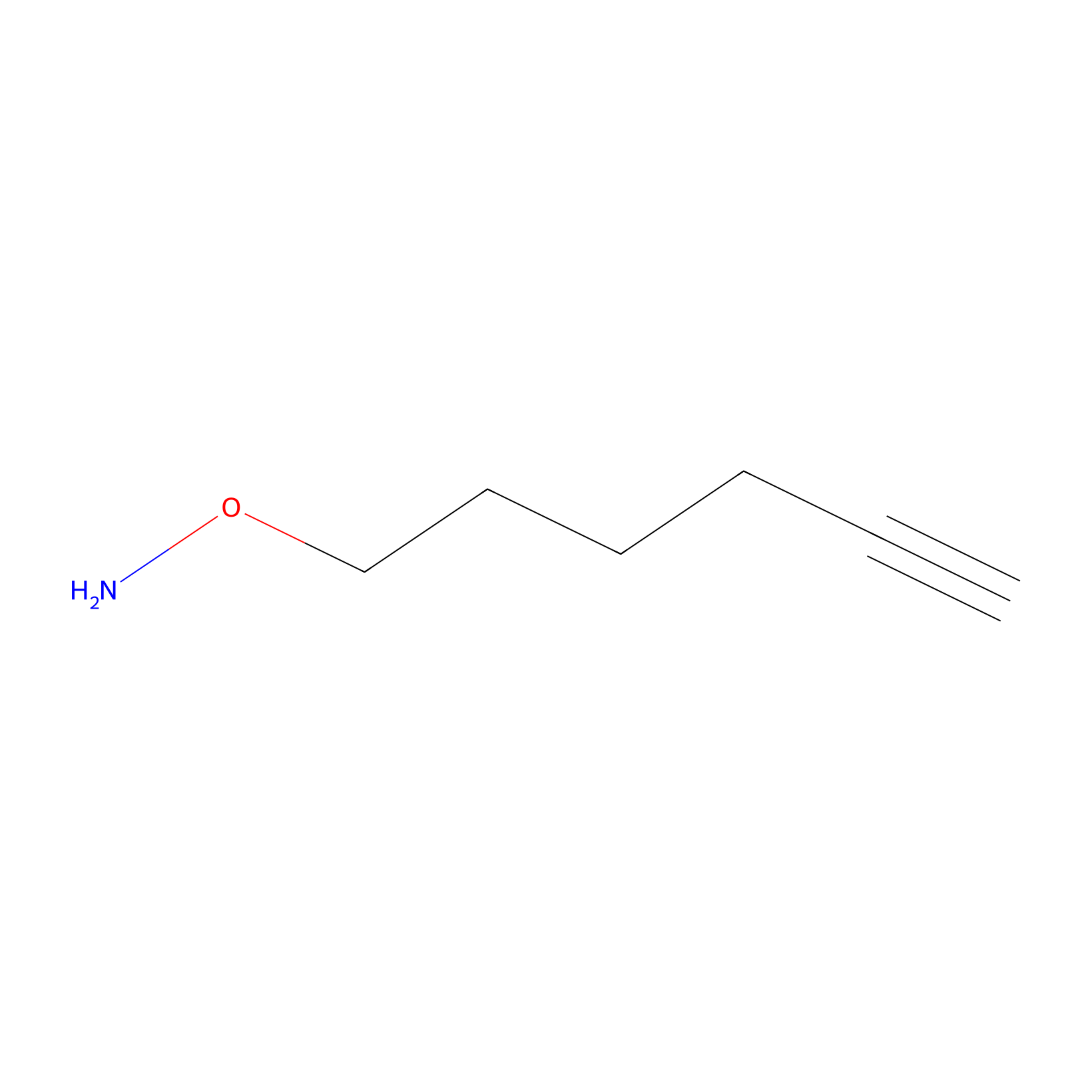 |
15.00 | LDD0443 | [4] | |
|
HHS-465 Probe Info |
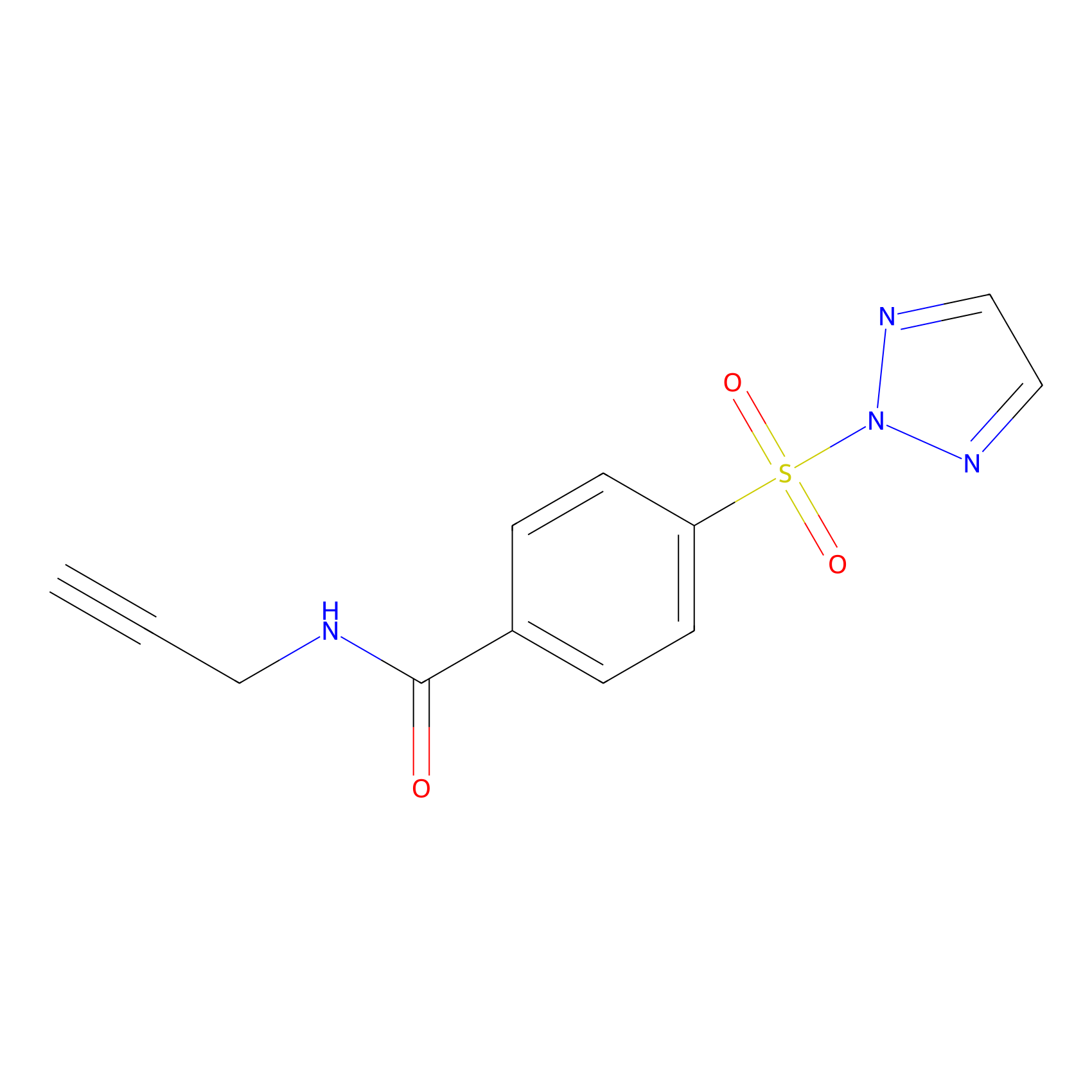 |
Y56(0.00); K49(0.00) | LDD2240 | [5] | |
Competitor(s) Related to This Target
| Competitor ID | Name | Cell line | Binding Site(Ratio) | Interaction ID | Ref |
|---|---|---|---|---|---|
| LDCM0156 | Aniline | NCI-H1299 | 13.04 | LDD0403 | [1] |
| LDCM0116 | HHS-0101 | DM93 | Y56(1.38); Y12(1.78) | LDD0264 | [2] |
| LDCM0117 | HHS-0201 | DM93 | Y12(1.06); Y43(1.12); Y56(2.75) | LDD0265 | [2] |
| LDCM0118 | HHS-0301 | DM93 | Y43(5.77); Y56(5.98) | LDD0266 | [2] |
| LDCM0119 | HHS-0401 | DM93 | Y12(0.66); Y56(2.02); Y43(11.86) | LDD0267 | [2] |
| LDCM0120 | HHS-0701 | DM93 | Y12(0.07); Y56(3.26); Y43(3.35) | LDD0268 | [2] |
The Interaction Atlas With This Target
The Protein(s) Related To This Target
Enzyme
References
